How to properly lubricate dental handpieces
12/29/2014 15:12
Dental handpieces belong to the group of medical devices that must be sterilized after each patient.
The main stages and rules of processing are defined in Methodological Letter dated March 21, 1995 N 12/20-208 “Organization of sanitary-hygienic and disinfection-sterilization regimes in dental institutions” and MU-287-113 dated December 30, 1998. With the abolition of SanPiN 2.1.3.2630-10 these regulations regulate the procedure and requirements for disinfection and sterilization of dental handpieces. Modern medical dental handpieces are a technically complex device that requires careful handling and care. This manual describes basic care and maintenance procedures for dental turbine and power handpieces. Following these recommendations will allow you to save money and time on their repair. This manual is common to all manufacturers. For more detailed information, please refer to your equipment manufacturer's documentation.
Sterility for materials and instruments
Most dental supplies are disposable and supplied in individual sealed packages. These are masks, gloves, napkins, brushes and applicators for applying varnishes and gels, tablets on which pastes are mixed, elements of saliva ejectors, etc.
Reusable instruments and materials undergo special treatment after each patient. It is performed in several stages:
- disinfection - soaking in solution for 2 hours;
- mechanical cleaning;
- washing (ultrasound can be used to remove all contaminants);
- washing in distilled water;
- drying;
- packaging in disposable sealed bags;
- dry heat or steam treatment (at high temperature).
After the sterilizer, the instrument remains in individual packaging, which is opened only before use in the presence of the patient.
Sanitary standards allow the use of conventional autoclaves in dental offices. In such equipment, superheated steam is supplied under pressure to sterilize accessories. The method is effective, but it does not always completely clean the pores on the surface of the instruments (if any). To increase the reliability of processing, dry heat sterilization is used, the temperature at which is so high that all microorganisms die. Another option is the use of automated autoclaves, with control of the operating protocol. To check the quality of cleaning, chemical indicators are additionally used - azopyram samples. If traces of biological material remain on surfaces, they show this. The results of such checks are recorded in a separate journal.
After sterilization and until use, reusable instruments are stored in special boxes with bactericidal irradiators. However, they are not removed from sealed bags.
Step 1: Disinfection of rotary dental instruments
Purpose: to destroy pathogenic and opportunistic microorganisms that contaminated products during medical procedures.
Disinfection of rotary dental instruments should be carried out immediately after their use, without allowing contaminants to dry on them, by completely immersing them in a disinfectant solution. The thickness of the mortar layer above the products should be at least 1 cm.
Working solutions for disinfecting instruments are prepared in advance. To do this, the required amount of concentrated disinfectant is mixed with water in the proportions specified in the instructions for use of the disinfectant.
For your information
To accurately dose the concentrate, you can use a graduated measuring cup or a disposable injection syringe.
All containers with working solutions of disinfectants must be equipped with tight-fitting lids, have clear inscriptions or labels indicating the product used, its concentration, purpose, preparation date, and expiration date of the solution.
To process dental instruments, special small-volume containers are used, which can significantly reduce the consumption of disinfectants.
Note!
To disinfect medical devices used in dentistry, it is necessary to use disinfectants that have a wide range of antimicrobial activity, including bactericidal, virucidal, and fungicidal properties.
The choice of modes is carried out between viruses or fungi of the genus Candida.
In tuberculosis medical organizations, regimens should be used in which disinfectant solutions are active against mycobacterium tuberculosis.
It is not recommended to use preparations containing aldehydes, since they are capable of fixing organic contaminants on the surface of instruments.
When processing steel and carbide burs, you should avoid using products containing hydrochloric acid and hydrogen peroxide - they can significantly deteriorate the properties of the tools.
Prolonged exposure of carbide and steel burs in the same container with a disinfectant solution can lead to their surface corrosion.
Treatment of dental offices
Cleaning regulations are determined by sanitary standards. All surfaces must be smooth, easy to clean, without microscopic pores or roughness. They are treated with disinfectants several times a day. Disinfection is carried out for furniture and equipment of walls, floors, ceilings, window sills, etc.
Dental offices must be equipped with special medical furniture. It is made from materials with surfaces that can withstand repeated disinfection and are free of micropores.
After each appointment, additional processing of all items touched by the doctor or patient is performed.
Some infections are transmitted by airborne droplets. To protect against them, dentistry can use bactericidal recirculating lamps (they continuously disinfect the air; they can operate in the presence of the patient), closed-cycle UV emitters, and special filters. A separate ventilation system is installed for surgical rooms. The air passes through fine filters. Access to the room must be through the sterilization department.
New requirements for dentists registered as individual entrepreneurs.
According to the requirements approved by Order No. 786n dated July 31, 2020, individual entrepreneurs will not be able to implement the equipment standard because do not have the right to hire specialized personnel (radiologists and x-ray laboratory technicians) and will not be able to obtain a sanitary and epidemiological certificate for working with sources of ionizing radiation.
To continue operating from January 1, 2022, all individual entrepreneurs will need to register a Limited Liability Company and obtain an X-ray license (license to operate in the field of using ionizing radiation sources).
To obtain an x-ray license you must:
- prepare a project for calculating the radiation protection of the X-ray room;
- purchase an X-ray machine and install it;
- obtain an expert opinion on activities with sources of ionizing radiation;
- obtain a technical passport for the X-ray room;
- obtain a sanitary and epidemiological certificate (SEZ) for activities with sources of ionizing radiation.
Dental equipment
Water supply systems. They are used to cool the tooth tissue during preparation. The jet is supplied through the tip. This treatment can only be carried out with distilled water. The design of the units is such that liquid from the oral cavity cannot enter the line even with a constant change in pressure.
Tips. During treatment, they come into contact with tooth tissue and require especially thorough cleaning. It becomes more complicated because the tips may have hidden cavities in which bacteria accumulate. For high-quality cleaning, the tip is blown twice: to remove mechanical impurities and rinse it with a hot antiseptic solution. Only after this is it sterilized in a disposable sealed bag.
Other tools. Endodontic accessories, burs, and scaler tips are stored in closed containers after treatment. You can only remove them from there with disposable sterile tweezers.
Individual dental kits. They include masks, gloves, aprons, and caps for the doctor and assistant. Special bags are used for waste materials. The standard set includes tips for a vacuum cleaner and a saliva ejector. If anesthesia is performed, the carpule and needle are disposable.
The described sterilization and disinfection protocol is used at the DentoSpas clinic and complies with the AntiAIDS and AntiHepatitis program. It ensures the safety of the patient during a dental appointment and eliminates infection and infection.
Cleaning, disinfection, lubrication, sterilization of dental handpieces
1. Preparation Disconnect the turbine handpiece from the adapter and remove the bur from the collet. Move turbines to a designated area with a clean environment. Remove organic contaminants with a paper towel.
2. Cleaning Manual: Clean the outer surface of the turbine with running water (<38˚C, demineralized water is recommended). Automatic: NSK turbine handpieces with this symbol on the body can be cleaned and sterilized in a thermal disinfector. When using the thermal disinfection device, follow the manufacturer's instructions.
NSK recommends using the Care3 Plus for automatic tip cleaning and lubrication. The device is easy to use and provides thorough lubrication regardless of operator skill and experience.
Easy to use:
| Open the door and carefully secure the lugs to the connectors (Up to 3 tips at a time). |
| Close the door tightly. Care3 Plus will not start if the door is not closed securely. |
| Select a cycle (Short, Long or Extra Long) for each tip. |
| Press the START button. Wait about two minutes for Care3 Plus to finish cleaning and lubricating. |
3. Disinfection Manual: Thoroughly wipe the outer surface of the turbine with a cleaning or disinfectant solution.
4. Lubrication It is recommended to lubricate the turbine tips twice a day - at the beginning and at the end of the day. If you continuously use the turbine for more than half an hour, lubrication is strongly recommended. Use an oil absorber to avoid airborne oil dust.
● Mandatory before sterilization ● Mandatory after each thermal disinfection
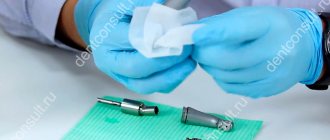
Manual: Lubricate the turbine with Pana Spray Plus / Pana Spray from NSK. To prevent oil from entering the environment, use an absorbent wipe. Remove excess grease.
Automatic: clean and lubricate turbines with Care3 Plus from NSK. Choose the right adapter for each turbine.
| A) Disconnect the turbine housing from the adapter. B) Insert the Phatelus Connector Spray nozzle into the tip and turn clockwise approximately 10 times. ● To prevent oil leakage, make sure the spray nozzle is securely seated in the tip. ● Use the PA spray nozzle to lubricate Pana Air series turbines. |
| C) Insert the nozzle into the rear of the turbine housing (connector side) and begin spraying. ● Make sure oil is coming out of the front of the head. ● If dirty oil comes out of the head, repeat spraying until clean oil comes out. ● The collet needs to be serviced. The recommended interval is once a week. |
5. Sterilization in an autoclave, class “B”. Now there are many manufacturers of medical autoclaves on the market, but there are only three special models for handpieces - the main one is Melaquick 12 +
If you have a regular autoclave in your clinic (and not a special one for handpieces), place the turbine tips in a sterilization bag and seal it. EN13060 4.6.3 recommends sterilization in an autoclave for 3 minutes (minimum holding time) at 134˚C or 15 minutes (minimum holding time) at 121˚C. NSK recommends Class B or S sterilization. All NSK turbines can be sterilized in an autoclave at temperatures up to 135˚C.
6. Storing handpieces after cleaning and lubrication: Immediately after the sterilization cycle, remove the turbine handpiece from the autoclave. Store the handpiece in a sterile, dust-free container or bring it to the treatment room for use.