The nerves of the eye are usually divided into three groups: motor, secretory and sensory.
Sensory nerves are responsible for regulating metabolic processes and also provide protection, warning of any external influences. For example, a foreign body entering the eye or an inflammatory process occurring inside the eye.
The task of the motor nerves is to ensure movement of the eyeball through coordinated tension of the motor muscles of the eye. They are responsible for the functioning of the dilator and sphincter of the pupil, and regulate the width of the palpebral fissure. The motor muscles of the eye, in their work to ensure the depth and volume of vision, are under the control of the oculomotor, abducens and trochlear nerves. The width of the palpebral fissure is controlled by the facial nerve.
The muscles of the pupil itself are controlled by nerve fibers in the autonomic nervous system.
Secretory fibers located in the facial nerve regulate the functions of the lacrimal gland of the organ of vision.
Diseases of peripheral processes
The main and most frequently diagnosed lesion of the peripheral plexuses, which is accompanied by functional disorders, is neuropathy or neuritis.
Symptoms characteristic of a violation of motor, sensory, and autonomic function occur. The person feels pain that radiates along the affected segment. Diseases that occur with dysfunction of the roots include: degenerative and dystrophic processes, metabolic disorders, inflammation of the roots and pinching by a tumor, osteophytes, hernia or other neoplasm.
Other peripheral plexus disorders:
- Optic nerves
- Diagnostics
- Cranial nerve examination
- Spinal cord, medulla spinalis. Development of the spinal cord.
- Neurology of motor nerves
- Second branch of the trigeminal nerve. Maxillary nerve, n. maxillaris. Pterygopalatine ganglion, ganglion pterygopalatinum.
- Vagus nerve (X pair, 10 pair of cranial nerves), n. vagus
- Functions of cranial nerves
- Taste nerves
- Treatment of the disease
- Diagnosis of neuralgia
- Sensitive nerve structures
- polyneuropathy, when several or many processes are involved in the lesion;
- plexitis is a disease characterized by damage to the entire plexus;
- radiculoneuritis is a simultaneous pathological process in the roots of the spinal canal and trunks;
- myeloradiculoneuritis - a condition characteristic of damage to the spinal cord, nerve trunks and roots;
- radiculitis – symptoms characteristic of injury to the spinal cord roots;
- ganglionitis is a pathological process characterized by damage to the intervertebral nodes.
Optic nerves
Visual disorders such as decreased acuity, color perception, visual field impairment, and blindness cause damage to any part of the second pair of the cranial nerve - visual. The types of violations depend on the dislocation of the source.
Tubular vision manifests itself in neuritis of the second pair of cranial nerves, hysteria, glaucoma, arachnoiditis. Complete blindness - as a result of injury, pathology of the chiasm. The condition is assessed based on the results of an examination of the visual components (acuity, visual field, etc.) and the fundus.
Polychromatic tables allow you to assess the level of color perception. Visual defects (so-called scotomas) detected by an ophthalmologist signal a pathology affecting the optic nerve. Patients themselves rarely consult a doctor when the severity decreases. Amblyopia can result in amaurosis. Sometimes complete loss of vision is the result of advanced pathology, when the pathological process is irreversible.
Diagnostics
Diagnosis is based on symptoms, medical history, and research:
- The doctor talks with the patient, finds out the symptoms. Finds out what the pain is and where it is located. Are there any infectious diseases in the head area? For example, otitis media, sinusitis, tooth extraction, adenoiditis.
- Then he examines and palpates the damaged area. The presence of swelling and redness is detected.
- When the doctor roughly presents the diagnosis, he examines for the presence of diseases with similar symptoms. That is, differential diagnosis is carried out. Refers for examination to a dentist and otolaryngologist.
- To determine the diagnosis, there is a method of applying a solution of cocaine hydroxide to the mucous membrane of the front of the nose. If the patient stops feeling pain, then this is inflammation of the nasociliary nerve.
- Next, instrumental studies should be carried out. Ophthalmoscopy, MRI of the head, biomicroscopy, anterior rhinoscopy.
- Once the diagnosis is made, treatment is prescribed.
With the help of additional examination, neuralgia of the nasociliary nerve is differentiated from other diseases. And they establish an accurate diagnosis.
Biomicroscopy of the eye
This is done using a slit lamp, the main part of which looks like a large slit. The device examines the structure of the eye, the back and front walls. This method allows you to see any damage, foreign body, and detect the disease at an early stage. Biomicroscopy allows you to see the condition of the retina and optic nerve.
Ophthalmoscopy
The fundus of the eye is examined using an ophthalmoscope. With the help of an eye mirror you can see blood vessels and nerves. Identify changes, redness, swelling.
Anterior rhinoscopy
This is done using a nasal speculum.
The anterior part of the nasal cavity is examined by inserting the device into the nostrils. The most suitable for this procedure is the Hartmann mirror. The doctor holds the device in his left hand, slightly dilating the nostril. In this case, the nasal septum and passages are visible. This is how redness, curvature, atrophy and hypertrophy are noticed. Sometimes it is possible to examine the nasal cavity using reflector lighting. It is used to examine children, as they may be frightened by an unknown object.
MRI of the head
To accurately confirm the diagnosis, an MRI of the head is performed. X-ray radiation is not used here. The examination is carried out using magnetic fields, which create an energy change in the examination area. This is how photographs are taken without the use of contrast. A cross-sectional image is displayed on the computer monitor.
First signs of damage
The first symptoms of nerve damage are discomfort in the gums, cheeks, and lower lip. Manifestations of the problem are:
- paresthesia, that is, a change in the level of sensitivity without pain;
- dysesthesia with pain in the affected area, a feeling of “pins and needles”, changes in the general sensitivity of the area;
- anesthesia - complete loss of sensation in a certain area.
In some cases, the lingual nerve, which runs from the side of the tongue into the gum tissue, may be affected. This is usually observed as a result of the removal of "eights" (in approximately 2.1% of all cases). When implanted, this nerve is affected less frequently. If this situation occurs, the following symptoms appear:
- salivation becomes profuse;
- involuntary biting of the tip of the tongue appears;
- diction disorders;
- burning sensation, numbness in the tongue;
- loss, change in taste;
- swallowing is impaired.
In 90% of cases, the problems go away on their own within seven to ten weeks; no special treatment is required.
Cranial nerve examination
Motor fibers control the muscles of mastication and some muscles of the middle ear.
The trigeminal nerve has three sensory nuclei, two of which are located in the medulla oblongata and pons, and one in the midbrain. The only motor nucleus of this nerve is located in the pons.
The name “trigeminal” is due to the fact that it consists of three branches carrying information from three “floors” of the face - the forehead; nose, cheeks and upper jaw; lower jaw. Motor fibers pass in the inferior branch of the trigeminal nerve.
Facial nerve
(VII pair) contains three types of fibers:
1) afferent sensory fibers bring impulses from the taste buds of the anterior two-thirds of the tongue. These fibers end in the nucleus of the solitary tract, the common sensory nucleus of the facial, glossopharyngeal and vagus nerves. It extends from the medulla oblongata into the pons;
2) somatic motor fibers innervate the facial muscles, as well as the muscles of the eyelids, and some muscles of the ear. These fibers come from the motor nucleus located in the pons;
3) autonomic parasympathetic fibers of the facial nerve innervate the submandibular and sublingual salivary glands, lacrimal glands, and glands of the nasal mucosa. They begin from the parasympathetic superior salivary nucleus, also located in the pons
Glossopharyngeal nerve
(IX pair) is similar in composition to the facial nerve, i.e. also contains three types of fibers:
1) afferent fibers bring information from the receptors of the posterior third of the tongue and end on the neurons of the nucleus of the solitary tract;
2) efferent somatic motor fibers innervate some muscles of the pharynx and larynx. The fibers begin in the nucleus ambiguus, the common motor nucleus for the glossopharyngeal and vagus nerves, located in the medulla oblongata;
3) efferent parasympathetic fibers begin in the inferior salivary nucleus and innervate the near-ear salivary gland.
Nervus vagus
(X pair) is so called because of the extensive distribution of its fibers.
It is the longest of the cranial nerves; with its branches it innervates the respiratory organs, a significant part of the digestive tract, and the heart. The Latin name for this nerve is p. vagus,
which is why it is often called the vagus.
Just like the VII and IX nerves, the vagus contains three types of fibers:
1) afferents carry information from the receptors of the previously mentioned internal organs and vessels of the chest and abdominal cavities, as well as from the dura mater of the brain and the external auditory canal with the auricle. These fibers carry information about the depth of breathing, pressure in blood vessels, stretching of organ walls, etc. They end in the nucleus of the solitary tract;
2) efferent somatic motor innervates the muscles of the pharynx, soft palate, and larynx (including those that control the tension of the vocal cords). The fibers begin in the double core;
Important Analysis of the activity of the autonomic nervous system
3) efferent parasympathetic fibers begin from the parasympathetic nucleus of the vagus nerve in the medulla oblongata. The parasympathetic part of the vagus nerve is very large, so it is predominantly an autonomic nerve.
From sensory cranial nerves
Only the vestibulo-auditory nerve (VIII pair) departs from the brain stem. It brings impulses from the auditory and vestibular receptors of the inner ear to the central nervous system. The sensory nuclei of this nerve - two auditory (ventral and dorsal) and four vestibular (lateral, medial, superior and inferior) - are located on the border of the medulla oblongata and the pons in the area of the vestibular field (see 7.2.2).
The VIII nerve originates in the inner ear and consists of two separate nerves - the cochlear (auditory) nerve and the vestibular (vestibular) nerve.
***
In conclusion, it should be noted that the nuclei of the cranial nerves have many afferents and efferents. Thus, all sensory nuclei send efferents to the thalamus (diencephalon), and from there information enters the cerebral cortex. In addition, sensory nuclei transmit signals to the reticular formation of the brain stem (see 7.2.6). All motor nuclei receive afferents from the cerebral cortex as part of the corticonuclear tract (see 6.4). Finally, there are numerous connections between the cranial nerve nuclei themselves, which facilitates the coordinated activity of various organs. In particular, thanks to the connections between the sensory and motor nuclei, the arcs of the stem unconditioned reflexes (for example, gag, blinking, salivation, etc.), similar to the spinal unconditioned reflexes, are closed.
Date added: 2015-10-01; | Copyright infringement
Related information:
Search on the site:
Trigeminal nerve
Trigeminal nerve: symptoms of inflammation and treatment methods
Inflammation of the trigeminal nerve does not threaten the patient’s life, but becomes a real test of fortitude.
The disease is accompanied by painful symptoms and significantly impairs the quality of life. Anxious anticipation of paroxysms of trigeminal neuralgia provokes depression, and some people experience suicidal thoughts. Insomnia is a typical condition for this pathology, which can be caused by both severe pain and deterioration of the emotional background. Among the accompanying changes are decreased performance, fatigue, and frequent headaches. The publication discusses the main causes of trigeminal neuralgia, clinical signs and features of diagnosing the disease, and the main methods of medical and surgical care. The possibilities and methods of treating trigeminal neuralgia using physiotherapeutic methods and folk remedies will be considered.
Trigeminal Nerve: Relationship Between Anatomy and Symptoms
The trigeminal facial nerve forms the fifth pair of cranial nerves (cranial nerves). It contains not only afferent sensory fibers, but also motor fibers. Sensory fibers provide superficial and proprioceptive (deep) sensitivity and transmit information to the brain from the skin of the entire face, mucous membranes of the eyes, nose and mouth, muscle and connective tissue structures, teeth and bones of the facial skeleton. Motor fibers go to the masticatory muscles.
Nervus trigeminus received its name due to the peculiarities of its anatomical structure. It is formed by three branches. The first is the orbital nerve. The second is called the maxillary nerve, and the third is the mandibular nerve.
The orbital, also the first branch of the nervus trigeminus, contains only sensory fibers. There are no motor neurons in its composition. The innervation zone includes the frontal zone, temples, eyebrow, upper eyelid, cornea and conjunctiva. Accordingly, with neuralgia of the ophthalmic branch, pain, numbness of the skin and paresthesia are localized in the forehead, eyebrows and eyelids. There may be a weakening or loss of reflexes, the adductor arc of which passes as part of the superior branch (suprabrow reflex, corneal reflex).
The second branch, like the first, contains exclusively sensory afferent fibers. The endings of sensory neurons are directed to the cheekbones and cheeks, wings and back of the nose, and lower eyelids. They also transmit signals from the mucous membrane of the nasal passages, maxillary bone, upper lip and upper teeth. With neuralgia of the second branch, pain, numbness of the skin and paresthesia are concentrated in the central part of the face on the right or left (the pain is always one-sided). Pain in the teeth of the upper row is typical.
The third branch, or mandibular nerve, contains not only sensory fibers, but motor (motor) neurons. This branch transmits information from the lower part of the face - the chin and mandibular bone, teeth, lower lip. Motor fibers transmit signals and coordinate the movements of many masticatory muscles and their antagonists. Also, the efferent fibers of the inferior branch go to the temporal muscle.
When the mandibular branch of the trigeminal nerve is affected, the epicenter of pain, skin numbness, hyperesthesia and paresthesia is located in the lower third. One of the symptoms is weakening or loss of the mandibular reflex. And since the lower branch also contains axons of motor neurons, an attack of neuralgia can be accompanied by motor disorders - spasm or paralysis of the muscles of the masticatory group and their antagonists.
Causes of trigeminal neuralgia
The pathogenesis of trigeminal neuralgia involves compression of one of its branches. The cause of root compression may be infection and inflammation, abnormal location or pathology of blood vessels; less commonly, the cause of compression is a tumor. Trigeminal neuralgia can be a symptom of multiple sclerosis. The following changes in the body and pathological conditions contribute to the development of inflammation of the trigeminal nerve:
- Hypothermia.
- Acute or chronic stress.
- Weakening of the immune system.
- Nervous fatigue and exhaustion.
- Hormonal imbalances.
- Chronic sinusitis, frontal sinusitis.
- Vascular pathology.
- Head injuries.
- The presence of foci of chronic infection in the body.
Very often, the true cause of trigeminal neuralgia is diseases of the oral cavity and teeth. In this regard, odontogenic inflammation of the trigeminal nerve is separately distinguished, which is secondary, and the cause of which is dental pathology. With single-gene neuralgia, as a rule, the maxillary or mandibular branch is affected, and among the symptoms of the disease there is a painful toothache.
Note. In 95% of cases with neuralgia n. trigeminus affects the 2nd or 3rd branches! This indicates a close connection between pathology and dental diseases.
Odontogenic neuralgia of the trigeminal nerve develops with the following dental diseases and anomalies of the development of the dentofacial system:
- Caries.
- Pulpitis.
- Periodontal disease, inflammation of periodontal tissue.
- Gingivitis.
- Osteomyelitis of the jaw bone.
- Retained and dystopic teeth.
- Poor quality dentures.
If you have inflammation of the trigeminal nerve, you must undergo an examination by a dentist to identify the above and other dental diseases. It is extremely important to sanitize foci of chronic infection - cure pulpitis, periodontitis, treat carious teeth, remove retained and dystopic teeth, replace low-quality orthopedic structures (prostheses).
For many patients, oral sanitation has helped to completely cure inflammation of the trigeminal nerve. It is also necessary to undergo an examination by an otolaryngologist, diagnose the paranasal sinuses, and treat chronic sinusitis or sinusitis.
Trigeminal nerve: clinical picture of the disease
The key clinical sign of inflammation of the trigeminal nerve is severe pain. The specifics of pain (localization, character) may differ, but one thing remains unchanged. The pain is always excruciating. Paroxysmal attacks “paralyze” and knock you out of the usual rhythm of life. They can be short-term, lasting no more than a few minutes, and the pain in this case is often shooting in nature. The second option is a constant burning, drilling or cutting pain that exhausts a person for 2-3 days.
Any manipulation on the face can provoke a paroxysm of neuralgic pain. Women, who for some unknown reason suffer much more often from this disease, often provoke a paroxysmal attack by applying decorative cosmetics, and men by shaving. The trigger factor for an attack of trigeminal pain can be even a normal conversation, washing with cool water, or hygiene procedures for caring for the oral cavity or facial skin.
Triggers are actions that provoke the return of paroxysmal pain. They are, as a rule, preceded by some events that happened shortly before that affected the general condition of the body. The true cause of exacerbation of inflammation of the trigeminal nerve can be hypothermia, exacerbation of herpes, colds, nervous fatigue and stress, even eating certain foods (fatty, spicy foods, chocolate, garlic).
The localization of pain depends on which branch is affected by the pathological process. With compression and inflammation of the orbital branch, pain in the upper third of the face dominates in the clinic; with damage to the middle root - in the upper jaw, upper teeth, and cheekbones. When the lower branch is compressed, the epicenter of pain is often localized in the teeth of the lower jaw. In addition, inflammation of the third branch is characterized by motor disturbances - spasm or paralysis of the masticatory muscles on the affected side.
Since the trigeminal nerve innervates one half of the face, pain, paresthesia and motor disorders are always unilateral. The patient complains of pain only on the right or only on the left. Because of this, slight or pronounced facial asymmetry often develops. With neuralgia of the lower branch, there may be a weakening of the bite on the affected side.
The trigeminal nerve contains sensory fibers that are part of the adductor arc of some reflexes. In this regard, with this disease, a decrease or loss of superciliary, corneal or mandibular reflexes is often observed. This symptom is detected during an examination of the patient by a neurologist.
Diagnostics
A preliminary diagnosis of inflammation of the trigeminal nerve is made by a neurologist based on the clinical picture, which includes:
- attacks of acute pain of a burning or shooting nature;
- unilateral localization of pain;
- numbness of the skin, tingling sensation or “pins and needles” on the affected side;
- weakening of reflexes;
- motor disorders with damage to the 3rd branch of the trigeminal nerve;
- vasomotor and secretory disorders (lacrimation, increased salivation).
To confirm the diagnosis, identify the cause of the disease, accurately determine the location of compression and differential diagnosis with other neurological diseases, instrumental examinations are prescribed:
- Computed tomography or MRI of the brain.
- X-ray of the facial skeleton.
- Angiography.
- Electromyography.
- Other.
Inflammation of the trigeminal nerve: treatment methods
The main objectives in the treatment of the trigeminal nerve are pain relief and complete cessation of paroxysms of neuralgic pain. To solve the problems, therapeutic and surgical approaches are used, as well as massage and physiotherapy. Treatment with folk remedies can be used as an additional, but not the main method.
Drug therapy
Drug treatment is carried out using strong painkillers, as well as anti-epileptic drugs. Carbamazepine, an anticonvulsant, helps relieve pain. Muscle relaxants and anti-neurotic drugs may be included in treatment regimens. In severe cases, narcotic analgesics are prescribed.
Painkillers from the NSAID group are ineffective for trigeminal neuralgia. Only the attending physician can prescribe strong analgesics and other drugs, and therefore the patient should contact a qualified specialist as soon as possible.
To reduce swelling and inflammation, hormones from the group of corticosteroids (Diprospan, Hydrocortisone) are included in the treatment protocols for the trigeminal nerve. Corticosteroids have a strong anti-exudative (anti-edematous) and anti-inflammatory effect, due to which rapid positive dynamics are achieved. Antihistamines can be used as an additional antiexudative and anti-inflammatory agent.
Neuroprotectors are also used in treatment to improve the nutrition of nerve cells and promote their recovery. If there are foci of chronic infection, antibiotics are prescribed. When exacerbation of labial herpes occurs, antiviral drugs are prescribed. Treatment regimens often include vitamin injections, vascular medications, antidepressants, tranquilizers and sedatives.
Rehabilitation of foci of chronic infection is mandatory. It is necessary to undergo diagnostics at the dentist, treat existing diseases of the teeth (caries, pulpitis), gums and periodontal tissues. If the diagnosis of neuralgia reveals chronic sinusitis, you need to undergo treatment from an otolaryngologist.
Surgery
In cases where the cause of paroxysmal attacks is compression of the root by a tumor or pathologically altered vessel, surgical treatment is performed. Its goal is to decompress nerve fibers, which is achieved by removing a tumor or moving a blood vessel. For decompression purposes, the nerve root can be isolated from surrounding tissues using a special protective sleeve.
If decompression is impossible or ineffective, surgical treatment has a different goal - complete cessation of impulse transmission along the fibers of the affected nerve. For this, a radiosurgery method can be used, which involves destruction of the sensory nerve, radiofrequency rhizotomy (destruction of the root using electromagnetic influence) or balloon compression.
Additional treatments
Additional methods of therapy include massage, physiotherapy and folk remedies. Massage improves blood circulation and helps reduce swelling, which increases nerve compression during inflammation. Physiotherapeutic procedures include electro- and phonophoresis, ultrasound, pulsed currents, and electromagnetic pulses. Reflexology can have a good effect.
At home, you can use the following traditional medicine recipes:
- Rinse your mouth with a decoction or infusion of chamomile. Rinsing has a minor effect in case of infectious etiology, but does not have an analgesic effect.
- Rubbing fir oil into the area of most severe pain. Can be repeated several times throughout the day to slightly reduce pain. It will not be possible to completely relieve the pain syndrome.
- Rubbing the skin of the affected area of the face with black radish juice. Has a weak analgesic effect.
- Healing clay: apply medicinal clay diluted in vinegar to the affected area. Used as an anti-inflammatory agent.
- Apply marshmallow infusion compresses to the painful area of the face for 30 minutes 1-2 times a day.
The trigeminal nerve is located deep, and traditional medicine cannot have a direct effect on inflamed and swollen tissues. The measures listed above are mainly distracting and auxiliary in nature; they cannot significantly alleviate the patient's condition. Therefore, if trigeminal neuralgia worsens, you should seek qualified help as soon as possible.
You can learn more about the methods of treating neuralgia and inflammation of the trigeminal nerve at the Galaktika clinic (Moscow).
Spinal cord, medulla spinalis. Development of the spinal cord.
As already noted, phylogenetically the spinal cord (trunk brain of the lancelet)
appears at stage III of nervous system development (tubular nervous system).
At this time, there is no brain yet, so the trunk brain has centers for controlling all processes of the body, both vegetative and animal (visceral and somatic centers)
. According to the segmental structure of the body, the trunk brain has a segmental structure; it consists of interconnected neuromeres, within which the simplest reflex arc is closed. The metameric structure of the spinal cord is preserved in humans, which determines the presence of short reflex arcs in humans.
With the appearance of the brain (cephalization stage)
Higher control centers for the entire body arise in it, and the spinal cord falls into a subordinate position. The spinal cord does not remain only a segmental apparatus, but also becomes a conductor of impulses from the periphery to the brain and back, and bilateral connections with the brain develop in it. Thus, in the process of evolution of the spinal cord, two apparatuses are formed: the older segmental apparatus of the spinal cord’s own connections and the newer suprasegmental apparatus of the bilateral pathways to the brain. This structural principle is also observed in humans.
Decisive factor in the formation of the trunk brain
is adaptation to the environment through movement. Therefore, the structure of the spinal cord reflects the way the animal moves. So, for example, in reptiles that do not have limbs and move with the help of the body (for example, a snake), the spinal cord is developed evenly along its entire length and has no thickenings. In animals that use limbs, two thickenings occur, and if the forelimbs are more developed (for example, the wings of birds), then the anterior (cervical) thickening of the spinal cord predominates; if the hind limbs are more developed (for example, the legs of an ostrich), then the posterior (lumbar) thickening is increased; if both forelimbs and hindlimbs (four-legged mammals) are involved in walking, then both thickenings are equally developed. In humans, due to the more complex activity of the hand as a labor organ, the cervical thickening of the spinal cord was differentiated more strongly than the lumbar one.
The noted phylogenetic factors play a role in the development of the spinal cord and ontogenesis
.
The spinal cord develops from the neural tube,
from its posterior segment (the brain arises from the anterior segment). From the ventral section of the tube, the anterior columns of the gray matter of the spinal cord (cell bodies of motor neurons), adjacent bundles of nerve fibers and processes of these neurons (motor roots) are formed. From the dorsal section arise the posterior columns of gray matter (cell bodies of interneurons), posterior funiculi (processes of sensory neurons).
Thus, the ventral part of the brain tube is primarily motor
, and the dorsal one is
primarily sensitive
.
The division into motor (motor)
and
sensory (sensitive)
areas extends throughout the neural tube and is maintained in the brain stem.
Due to the reduction of the caudal part of the spinal cord, a thin cord of nervous tissue is obtained, the future filum terminale.
Initially, in the 3rd month of uterine life, the spinal cord occupies the entire spinal canal, then the spine begins to grow faster than the brain, as a result of which the end of the latter gradually moves upward (cranially). At birth, the end of the spinal cord is already at the level of the third lumbar vertebra, and in an adult it reaches the height of the first - second lumbar vertebra. Thanks to this “ascent” of the spinal cord, the nerve roots extending from it take an oblique direction.
Anatomy
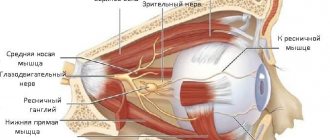
Numerous nerves of the eye are busy ensuring the proper functioning of the organ of vision and protecting it from external influences. In addition, they make it possible to operate the auxiliary apparatus of the eye, carrying out the necessary precise regulation of all the underlying functions.
The nerves of the eye are divided into several groups in terms of species: sensory, motor, secretory nerves.
Sensitive nerves regulate metabolic processes and protect, warning of any external influence, for example, penetration of foreign bodies into the cornea, or an inflammatory process in the eye, for example, iridocyclitis. The main role in providing eye sensitivity belongs to the trigeminal nerve.
Motor nerves make possible movements of the eyeball through tension of the extraocular muscles, as well as the action of the pupillary sphincter and dilator. In addition, they change the width of the palpebral fissure. In their work, while providing depth and volume of vision, the extraocular muscles control the oculomotor, abducens and trochlear nerves. The facial nerve regulates the possible width of the palpebral fissure. Nerve fibers belonging to the autonomic nervous system control the muscles of the pupil.
Secretory fibers are part of the facial nerve and, above all, regulate the functioning of the lacrimal gland.
Structure of the nervous system of the eye
All nerves involved in the functioning of the eye originate from nerve cells in the brain or nerve ganglia. The nervous system ensures the functioning of muscles, the tone of blood vessels, the speed of metabolic processes, the sensitivity of the eye, as well as its auxiliary apparatus.
Five of the twelve pairs of cranial nerves take part in the innervation of the eye, these are: oculomotor, trochlear, abducens, facial, and trigeminal nerves.
Nerve cells of the brain serve as the basis for the oculomotor nerve, which in turn has a close connection with the nerve cells of the trochlear and abducens nerves, as well as the spinal cord, auditory and facial nerves. Due to this, there is a coordinated reaction of the eyes, together with the head and torso, to visual and auditory stimuli, as well as to changes in body position.
The oculomotor nerve enters the orbit through the superior orbital fissure, providing action to the levator palpebrae superioris muscle, as well as the inferior, superior, inferior oblique and internal rectus muscles. At the same time, the oculomotor nerve includes branches that ensure the functioning of the ciliary muscle, as well as the sphincter of the pupil.
The abducens and trochlear nerves enter the orbit in the same way - through the superior orbital fissure, their function is to innervate the superior oblique and external rectus muscles, respectively.
The facial nerve includes not only motor fibers of the nerves, but also branches that regulate the functioning of the lacrimal gland. It causes numerous facial muscles to move, including the orbicularis oculi muscle.
The trigeminal nerve includes autonomic fibers and is mixed; it regulates muscle function as well as sensitivity. True to its name, the trigeminal nerve is divided into three large branches:
- The first branch is the optic nerve. It enters the orbit through the superior orbital fissure and is divided into three main nerves: nasociliary, frontal, and lacrimal.
- The nasolacrimal nerve is localized in the muscular infundibulum, dividing into posterior and anterior ethmoidal branches, long ciliary branches, and nasal branches. In addition, it gives off a connecting branch to the ciliary ganglion of the eye. The ethmoidal nerves as part of the nasolacrimal nerve provide sensitivity to the cellular component of the ethmoidal labyrinth, the nasal cavity, the skin of the wings, and the tip of the nose.
- Long ciliary nerves passing through the sclera in the optic nerve zone are directed into the supravascular space into the anterior segment of the eye, where, together with short ciliary nerves extending from the ciliary ganglion, they form the nerve plexus of the ciliary body and the surrounding area of the cornea. This nerve plexus is responsible for sensitivity and regulation of metabolic processes in the anterior segment of the eye. At the same time, the long ciliary nerves include sympathetic nerve fibers that extend from the nerve plexus at the internal carotid artery, regulating the work of the pupillary dilator.
The origin of the short ciliary nerves is the ciliary ganglion; they pass through the sclera, bending around the optic nerve, and provide innervation to the choroid. The ciliary or ciliary nerve ganglion unites nerve cells involved in sensory (via the nasociliary root), motor (via the oculomotor root); autonomic (using sympathetic nerve fibers) innervation of the eyeball. The ciliary node is located under the rectus externus muscle, 7 mm posterior to the eyeball area, and is in contact with the optic nerve. The long and short ciliary nerves, in turn, jointly regulate the work of the pupillary sphincter and dilator; sensitivity of the cornea, ciliary body, iris; tone of blood vessels; metabolic processes of the eyeball. The subtrochlear nerve is the last branch of the nasociliary nerve and provides sensory innervation to the dermis of the root of the nose, the inner corners of the eyelids and a small area of the conjunctiva.
- The frontal nerve, entering the orbit, splits into two branches: the supraorbital nerve and the supratrochlear nerve, which provide sensitivity to the skin in the middle region of the upper eyelid and the frontal zone.
- The lacrimal nerve divides in the orbit into a superior and inferior branch. The superior branch makes possible the work of the lacrimal gland, provides sensitivity to the conjunctiva, as well as the skin at the outer corner of the eye and the area of the upper eyelid. When the lower branch of the lacrimal nerve is connected to the zygomaticotemporal nerve (its branch), sensitivity of the skin in the zygomatic region is ensured.
- The second branch is the maxillary nerve. It is divided into two main branches - the infraorbital branch and the zygomatic branch, which provides nervous regulation of the auxiliary organs: the middle of the lower eyelid, the upper half of the lacrimal duct, the lower half of the lacrimal sac, the skin of the forehead, the skin of the zygomatic region.
- The third branch, arising from the trigeminal nerve, does not participate in the innervation of the eye.
Methods for diagnosing diseases of the optic nerves
- External examination, determining the width of the palpebral fissure and the position of the upper eyelid.
- Inspection of the ability to move the eyeball, checking the functioning of the extraocular muscles.
- A measurement of pupil size that includes the direct and concomitant reaction of the pupil to light.
- Inspection of skin sensitivity, according to the areas of innervation by the corresponding nerves.
- Palpation for tenderness of the trigeminal nerve exit points.
Signs of eye nerve diseases
- Marcus-Gunn syndrome.
- Paralysis and paresis of the extraocular muscles.
- Horner's syndrome.
- Paralytic strabismus.
- Pto-upper century.
- Dysfunction of the lacrimal glands.
- Trigeminal neuralgia.
Neurology of motor nerves
If cranial nerves are affected, neurological manifestations depend on their functional purpose. The group of motor ones emanating from the parasympathetic nuclei of the trunk includes:
- oculomotor (III);
- block (IV);
- abducent (VI);
- additional (XI);
- sublingual (XII).
Oculomotor pair
from the orbit it controls the oblique muscle, which ensures the elevation of the eyelid. The oculomotor provides neurons to the muscle that controls the pupil and eyelash movements. If it is affected, weakness and even paralysis of the above muscles appear. Then the eye opens only partially or does not open at all, accommodation is disrupted, the light reaction of the pupil disappears, and strabismus may develop.
Block
from the skull it runs into the eye socket and controls the rotation of the eyeball with the help of the oblique muscle. Its defeat is accompanied by deviations of the apple of the affected eye and double vision. The fibers of the trigeminal nerve branch into processes and perform many functions. They control and provide sensitivity to various parts of the face.
Neuronal bundle diverting
directs contractions on one side of the lateral muscle, and on the opposite side, the medial muscle. The symptoms that appear depend on the degree of damage to the nuclei or neurons. Various visual disturbances may occur: strabismus, double vision, hemiplegia. The greater length of the nerve increases the risk of damage.
Additional
consists of 2 (spinal and vagus) parts. Through a conductive (two-neuron pathway) it carries out motor functions. When the nerve is irritated, tonic muscle spasms develop in the sternoclavicular area (nodding movements of the head, its unnatural rotation). A one-sided lesion leads to difficulties in moving the head and shoulders; a bilateral lesion leads to the inability to make these movements, often the head tilts back.
Hyoid nucleus
located in the rhomboid fossa, its motor filaments innervate the lingual muscles. Damage to the 12th nerve leads to their paresis and decreased tongue mobility. Bilateral damage is fraught with the development of paralysis of the innervated organ (glossoplegia).
Important Delusional disorder
Vagus nerve (X pair, 10 pair of cranial nerves), n. vagus
N. vagus, vagus nerve
, which developed from the 4th and subsequent gill arches, is so called due to the vastness of its distribution.
It is the longest of the cranial nerves. With its branches, the vagus nerve supplies the respiratory organs, a significant part of the digestive tract (up to the colon sigmoideum)
, and also gives branches to the heart, which receives fibers from it that slow down the heartbeat.
N. vagus
contains three types of fibers:
1. Afferent (sensory) fibers
, coming from the receptors of the named viscera and vessels, as well as from some part of the dura mater of the brain and the external auditory canal with the auricle to the
sensitive nucleus (nucleus solitarius)
.
2. Efferent (motor) fibers
for the voluntary muscles of the pharynx, soft palate and larynx and the efferent (proprioceptive) fibers emanating from the receptors of these muscles.
These muscles receive fibers from the motor nucleus (nucleus ambiguus)
.
3. Efferent (parasympathetic) fibers
emanating from
the vegetative nucleus (nucleus dorsalis n. vagi)
.
They go to the myocardium of the heart (slow down the heartbeat) and the muscular lining of the blood vessels (dilate the blood vessels). In addition, the cardiac branches of the vagus nerve include the so-called n. depressor, which serves as a sensory nerve for the heart itself and the initial part of the aorta and is in charge of the reflex regulation of blood pressure. Parasympathetic fibers also innervate the trachea and lungs (narrow the bronchi), esophagus, stomach and intestines to the colon sigmoideum
(increase peristalsis), embedded in the named organs of the gland and glands of the abdominal cavity - liver, pancreas (secretory fibers), kidneys.
Parasympathetic part of the vagus nerve
is very large, as a result of which it is primarily an autonomic nerve, important for the vital functions of the body. The vagus nerve is a complex system consisting not only of nerve conductors of heterogeneous origin, but also containing intra-trunk nerve nodes.
Fibers of all types associated with the three main nuclei of the vagus nerve
, leave the medulla oblongata in its sulcus lateralis posterior, below the lingual tray nerve, with 10-15 roots, which form a thick nerve trunk, leaving the cranial cavity together with the lingual tray and accessory nerves through the foramen jugulare.
In the jugular foramen, the sensitive part of the nerve forms a small node - ganglion superius
, and at the exit from the foramen - another fusiform ganglion thickening -
ganglion inferius
.
Both nodes contain pseudounipolar cells, the peripheral processes of which are part of the sensory branches going to the named nodes or receptors of the viscera and blood vessels ( ganglion inferius
) and the external auditory canal (
ganglion superius
), and the central ones are grouped into a single bundle, which ends in
the sensitive nucleus, nucleus solitarius.
Upon exiting the cranial cavity, the trunk of the vagus nerve
goes down to the neck behind the vessels in the groove, first between v.
jugularis interna and a. carotis interna, and below - between the same vein and a. carotis communis, and it lies in the same vagina with the named vessels. Next, the vagus nerve penetrates through the upper thoracic aperture into the chest cavity, where its right trunk is located in front of a. subclavia, and the left one is on the anterior side of the aortic arch. Going down, both vagus nerves go around the root of the lung from behind on both sides and accompany the esophagus, forming plexuses on its walls, with the left nerve passing along the front side, and the right one along the back. Together with the esophagus, both vagus nerves penetrate through the hiatus esophageus of the diaphragm into the abdominal cavity, where they form plexuses on the walls of the stomach. The trunks of the vagus nerves
in the uterine period are located symmetrically on the sides of the esophagus.
After the stomach turns from left to right, the left vagus moves forward, and the right one moves back, as a result of which the left vagus
, and the right one on the posterior surface.
content .. 189 190 191 192 193 194 195 196 197 198 199 ..
Maxillary nerve (human anatomy)
Maxillary nerve
, n. maxillaris, the second branch of the trigeminal nerve, it is mainly sensory. Has a thickness of 2.5-4.5 mm; consists of 25-70 small bundles containing from 30,000 to 80,000 papillary nerve fibers of predominantly small diameter (up to 5 microns).
The maxillary nerve innervates the dura mater, the skin of the lower eyelid, the outer corner of the eye, the anterior part of the temporal region, the upper part of the cheek, the wings of the nose, the skin and mucous membrane of the upper lip, the mucous membrane of the posterior and lower parts of the nasal cavity, the mucous membrane of the sphenoid sinus, palate, dental organs and teeth of the upper jaw. Upon exiting the skull through the foramen rotundum, the nerve enters the pterygopalatine fossa, passes from back to front and from inside to outside. The length of the segment and its position in the fossa are related to the shape of the skull. With brachycephaly, the length of the nerve segment in the fossa is 15-22 mm; it is located deep in the fossa - up to 5 cm from the middle of the zygomatic arch. Sometimes the nerve in the pterygopalatine fossa is covered by a bone crest. With dolichocephaly, the length of the nerve section in question is 10-15 mm and it is located more superficially - up to 4 cm from the middle of the zygomatic arch.
Within the pterygopalatine fossa, the maxillary nerve gives off the ramus meningeus to the dura mater and is divided into three branches: 1) pterygopalatine nerves, nn. pterygopalatini, going to gangl. pterygopalatinum; 2) zygomatic nerve, n. zygomaticus; 3) inferior orbital nerve, n. infraorbitals, which is a direct continuation of the maxillary nerve (Fig. 232, 233).
Rice. 232. Scheme of the structure of the maxillary nerve
1. Pterygopalatine nerves, nn. ptery go palatini, very variable in number (1-7) and length (9-30 mm); depart from the maxillary nerve at a distance of 1-2.5 mm from the round foramen and go to the pterygopalatine ganglion, giving sensory fibers to the nerves starting from the ganglion. Some pterygopalatine nerves bypass the ganglion and join its branches.
Pterygopalatine ganglion, gangl. pterygopalatinum, - the formation of the parasympathetic part of the autonomic nervous system. The node is triangular in shape, 3-5 mm long, contains multipolar cells and has three roots: a) sensitive - nn. pterygopalatine b) parasympathetic - greater petrosal nerve, n. petrosus major, a branch of the intermediate nerve that carries secretory fibers to the lacrimal gland, to the glands of the nasal cavity and palate; c) sympathetic - deep petrosal nerve, n. petrosus profundus, a branch of the plexus caroticus internus, containing postganglionic sympathetic nerve fibers from the cervical ganglia. Branches extend from the node, including secretory (parasympathetic and sympathetic) and sensory fibers: orbital branches, rami orbitales; posterior superior nasal branches, rami nasales posteriores superiores; palatine nerves, nn. palatini (see Fig. 233).
Rice. 233. Olfactory nerve, pterygopalatine ganglion and branches of the trigeminal nerve. 1 - lower nasal passage; 2, 4, 7 - lower, middle and upper turbinates; 3 - middle nasal passage; 5 - olfactory bulb; 6 - olfactory nerves; 8 - sphenoid sinus; 9 - optic nerve; 10, 23 - internal carotid artery; 11 - oculomotor nerve; 12 - pterygopalatine node; 13 - orbital nerve; 14 - maxillary nerve; 15 - trigeminal node; 16 - nerve of the pterygoid canal; 17 - trigeminal nerve; 18 - greater petrosal nerve; 19 - deep petrosal nerve; 20, 31 - facial nerve; 21 - vestibulocochlear nerve; 22 - internal carotid nerve plexus; 24 - lingual nerve; 25 - lower alveolar nerve; 26 - drum string; 27 - middle artery of the meninges; 28 - maxillary artery; 29 - styloid process; 30 - mastoid process; 32 - parotid salivary gland; 33 - perpendicular plate of the palatine bone; 34 - medial pterygoid muscle; 35 - palatine nerves; 36 - soft palate; 37 - hard palate; 38 - upper lip
The orbital branches, rami orbitales, in the amount of 2-3 thin stems, penetrate through the lower orbital fissure into the orbit and further along with n. ethmoidalis posterior go through small openings in the sutura sphenoethmoidalis to the mucous membrane of the posterior cells of the ethmoid labyrinth and the sphenoid sinus.
The posterior superior nasal branches, rami nasales posteriores superiores, in the number of 8-14 stems, emerge from the pterygopalatine fossa through the foramen sphenopalatinum into the nasal cavity, concentrated in two groups: lateral and medial. The lateral branches, rami nasales posteriores superiores laterales (6-10 stems), go to the mucous membrane of the posterior sections of the superior and middle nasal concha and nasal passages, the posterior cells of the ethmoid sinus, the upper surface of the choanae and the pharyngeal opening of the auditory tube. The medial branches (2-3 trunks) branch in the mucous membrane of the upper part of the nasal septum. One of the medial branches is the nasopalatine nerve, n. nasopalatinus, passes between the periosteum and the mucous membrane of the septum together with a. nasalis posterior septi forward to the nasal opening canalis incisivus, through which it reaches the mucous membrane of the anterior part of the palate. Forms a connection with ramus nasalis n. alveolaris superior.
Palatine nerves, nn. palatini, spread from the node through the canalis palatinus major, forming three groups of nerves: a) greater palatine nerve, n. palatinus major; b) small palatine nerves, nn. palatini minores; c) lower posterior lateral nasal branches, rami nasales posteriores inferiores laterales.
Greater palatine nerve, n. palatinus major, the thickest branch, exits through the foramen palatinum majus to the palate, where it splits into 3-4 branches that innervate most of the mucous membrane of the palate and its glands in the area from the canines to the soft palate.
Lesser palatine nerves, nn. palatini minores, enter the oral cavity through the small palatine openings, branch in the mucous membrane of the soft palate and the region of the palatine tonsil, as well as in m. levator veli palatini (motor fibers come from n. facialis through n. petrosus major).
The lower posterior lateral nasal branches, rami nasales posteriores inferiores laterales, enter the canalis palatinus majus, leave it through small openings and, at the level of the inferior nasal concha, penetrate the nasal cavity, innervating the mucous membrane of the inferior concha, the middle and lower nasal passages and the maxillary sinus.
2. Zygomatic nerve, n. zygomaticus, branches off from the maxillary nerve within the pterygopalatine fossa and penetrates through the inferior orbital fissure into the orbit, where it runs along its outer wall and exits through the foramen zygomaticoorbitale, dividing into two branches.
The zygomaticofacial branch, ramus zygomaticofacialis, exits through the foramen zygomaticofaciale onto the anterior surface of the zygomatic bone, branches in the skin of the upper part of the cheek and the region of the outer canthus. Gives a connecting branch to n. facialis.
The zygomaticotemporal branch, ramus zygomaticotemporalis, leaves the orbit through the opening of the same name in the zygomatic bone, pierces the temporal muscle and its fascia, and innervates the skin of the anterior part of the temporal and posterior part of the frontal regions. Gives a connecting branch to n. lacrimalis, sending secretory parasympathetic fibers to the lacrimal gland.
3. Inferior orbital nerve, n. infraorbitalis, is a continuation of the maxillary nerve, receiving its name from the origin of the last branches mentioned above. The inferior orbital nerve leaves the pterygopalatine fossa through the inferior orbital fissure, lies together with the vessels of the same name on the lower wall of the orbit in the sulcus infraorbitalis (in 15% of cases there is a bone canal instead of a groove) and exits through the foramen infraorbitale under the quadratus labii muscle, dividing into terminal branches . The length of the inferior orbital nerve varies. In brachycephals, the nerve trunk is 20-27 mm, and in dolichocephals it is 27-32 mm. The position of the nerve in the orbit corresponds to the parasagittal plane, drawn through the sutura infraorbitalis. The nature of the origin of the branches can also be different: scattered, in which numerous thin nerves with a large number of connections depart from the trunk, or main with a small number of large nerves. Along its path, the infraorbital nerve gives off the following nerves:
Superior alveolar nerves, nn. alveolares superiores, innervating the teeth and upper jaw. The following branches of the superior alveolar nerves are distinguished: a) posterior, b) middle, c) anterior (Fig. 234).
Rice. 234. Maxillary nerve. 1 - posterior upper alveolar branches; 2 - zygomatic nerve; 3 - maxillary nerve; 4 - nerve of the pterygoid canal; 5 - orbital nerve; 6 - trigeminal nerve; 7 - mandibular nerve; 8 - drum string; 9 - ear node; 10 - connecting branches of the pterygopalatine ganglion with the maxillary nerve; 11 - chewing nerve; 12 - inferior alveolar nerve; 13 - lingual nerve; 14 - pterygopalatine node; 15 - inferior orbital nerve; 16 - anterior upper alveolar branches
The posterior superior alveolar branches, rami alveolares superiores posteriores, branch from the lower orbital nerve, usually in the pterygopalatine fossa in an amount of 4 to 8 and spread together with the vessels of the same name along the surface of the tubercle of the upper jaw. Part of the most posterior nerves runs along the outer surface of the tubercle down to the alveolar process. The rest enter through the foramina alveolaria posteriora into the canalis alveolaris, from which they exit onto the outer surface and into the bone canaliculi of the upper jaw, forming with other upper alveolar branches the superior dental plexus, plexus dentalis superior. The plexus lies in the alveolar process of the upper jaw above the apices of the roots; it is quite dense, broadly looped, stretched along the entire length of the alveolar process. From the plexus extend the upper gingival branches, rami gingivales superiores, to the periodontium and periodontium, i.e., to the mucous membrane of the alveolar process, gum and socket in the area of the upper molars, and the upper dental branches, rami dentales superiores, to the apices of the roots and foramina apicalia of the large molars, in the pulp cavity of which they branch. In addition, the posterior superior alveolar branches send thin nerves to the mucous membrane of the maxillary sinus.
The middle superior alveolar branch, ramus alveolaris superior medius, in the form of a stem, rarely 2, branches from the inferoorbital nerve, often in the pterygopalatine fossa and less often within the orbit, passes in one of the alveolar canals and branches in the bone canaliculi of the upper jaw as part of the plexus dentalis superior. It has connecting branches with posterior and anterior superior alveolar branches. Innervates the periodontium and periodontium through the upper desial branches in the area of the upper premolars and the upper dental branches - the upper premolars.
The anterior superior lunate branches, rami alveolares superiores anteriores, usually in the amount of 1-2 trunks, rarely 3, arise from the inferoorbital nerve in the anterior part of the orbit; they leave it through the foramina alveolaria anteriora and exit through the canalis alveolaris onto the anterior surface of the upper jaw, where they form part of the plexus dentalis superior. The mucous membrane of the alveolar process, gums and sockets in the area of the upper canines and incisors and the upper dental branches - the upper canines and incisors - are innervated by means of the upper desi branches. In addition, the anterior superior alveolar branches send a thin nasal branch to the mucous membrane of the anterior nasal cavity.
2. The lower eyelid branches, rami palpebrales inferiores, branch from the lower orbital nerve at the exit from the foramen infraorbitale, penetrate through the quadratus muscle of the upper lip and, branching, innervate the skin of the lower eyelid.
3. The external nasal branches, rami nasales externi, innervate the skin in the area of the wing of the nose.
4. The internal nasal branches, rami nasales interni, supply the mucous membrane of the nasal vestibule.
5. The superior labial branches, rami labiates superiores, in the amount of 3-4 stems, run down between the upper jaw and the quadratus muscle of the upper lip, innervating the skin and mucous membrane of the upper lip to the corner of the mouth. All of the listed external branches of the inferior orbital nerve form connections with the branches of the facial nerve.
content .. 189 190 191 192 193 194 195 196 197 198 199 ..
Functions of cranial nerves
There are 3 types of structures considered. Some are responsible for muscle contraction; these are the motor (activator) functions of the cranial nerves. Others transmit impulses and “knowledge” received from the senses to the cortex for analysis. There are also mixed cranial nerves that perform both tasks in parallel. Functionality is determined by the type of neuronal fiber transmission.
Motor cranial nerves
There are 4 pairs in this group, each performing separate tasks. Motor nerves:
- Trochlear (IV)
– associated with the upper muscle, facial (oblique). Provides the eyeball with the ability to rotate to the sides and rotate. - Abductor (VI)
– connected to the rectus lateralis muscle. Necessary for retracting the eyeball to the desired direction. - Accessory (XI)
- innervates the sternocleidomastoid muscle. Thanks to it, the neck bends, the head turns, tilts to the sides, leans back, and the shoulders move. - Hypoglossal (XII)
– the 12th pair of cranial nerves is connected to the oral cavity. The structure is primarily responsible for comfortable swallowing and precise movements of the tongue muscle.
Important Assessment of neurological status in emergency care
Sensory cranial nerves
An alternative name is sensory pairs, due to their connections with the sensory organs. Sensory cranial nerves perform the following functions:
- Olfactory (I)
– the shortest fibers in length. Necessary for the perception of smells. - Visual (II)
– transports impulse data from the photoreceptors of the retina to the cortex. These cranial nerves are responsible for visualization. - Vestibulocochlear (VIII)
– vestibular functions. This pair is necessary to maintain a sense of balance and transmit auditory signals.
Mixed cranial nerves
The described group of neuron fibers is responsible for both motor activity and the sensitivity of certain structures. Cranial mixed nerves:
- Oculomotor (III)
– transmits signals to the middle section. The pair is responsible for the sensitivity of the pupils to changes in light (constriction and dilation). At the same time, nerves provide movement of the eyeballs. - Trigeminal (V)
is the largest neural formation. This pair transmits sensory information from facial tissues and mucous membranes. Additionally, the structures regulate the movements of the masticatory muscles. - Facial (VII)
– the main task is to “command” facial expressions, control the functioning of the salivary and lacrimal glands. In parallel, the nerves transmit information about taste from the tongue receptors to the brain. - Glossopharyngeal (IX)
– associated with structures of the same name. In the oral cavity, these human cranial nerves collect sensory information about taste. The pair also provides swallowing by transmitting commands to the cervical muscles and the salivary gland. - The vagus (X)
is the most “loaded” nerve. “Serves” the heart, respiratory tract, digestive and filtering organs. It affects the swallowing process, regulates the overall activity of a person, and adjusts the intensity of stress. The pair can interact with the sympathetic system and most internal organs.
Functions of the infraorbital nerve
The infraorbital nerve performs important functions.
The structure and location of the network of nerve endings and branches of the infraorbital nerve determines the functions it performs.
Each small branch is involved in providing nerve endings to a separate area of the human face.
All teeth in the upper jaw are supplied (innervated) by branches of the infraorbital trunk: large molars - with the help of the posterior upper alveolar branches, small teeth - with the help of the middle branches, incisors and canines - with the help of the anterior branches.
The upper gingival and dental branches depart from the upper alveolar nerve trunks, which innervate the teeth. The anterior superior alveolar branches are partially involved in the innervation of the nasal mucosa, and the posterior ones - the mucous membrane of the maxillary cavity.
The remaining branches of the infraorbital nerve supply the corresponding parts of the face with nerve endings:
- Skin of the nose - external nasal branches.
- Nasal mucosa - internal nasal branches.
- The skin of the lower eyelid - the lower branches of the eyelids.
- The skin and mucous membrane of the upper lip - the upper labial branches.
The infraorbital nerve, unlike the trigeminal nerve, has only sensory roots. Thus, the innervation of the teeth of the upper jaw, skin and mucous membrane of the mouth, nose allows a person to feel changes in temperature and humidity, touching these surfaces, and partly the relative relative position of individual parts of the face.
Taste nerves
Ellipsoidal-shaped cells located in the mouth are designed to detect taste (taste bud). Taste buds consist of basal cells and chemoreceptors (the taste receptors themselves). Taste buds of different types are located on the tongue, cheeks, soft palate, epiglottis in the initial part of the esophagus.
Sensations of sweet, salty, bitter are transmitted to a certain part of the GM cortex through the nerves: glossopharyngeal (IX), facial (YII), vagus (YIII). Taste bioimpulses travel through nerve fibers to the thalamus, then to the guardian region of the GM cortex.
Damage to these nerves and the central parts of the brain (tumors, injuries, operations) provokes neural taste disorders:
- total ageusia, loss of the ability to distinguish taste (salty, sweet, etc.);
- ageusia partial;
- specific ageusia;
- dysgeusia (perverted taste).
Treatment of the disease
Treatment of nasociliary nerve neurosis consists of eliminating the disease that affects it.
In case of inflammatory processes in the ENT organs, patients are prescribed a course of treatment with antibiotics and a set of drugs that affect the elimination of inflammation and restoration of the mucous membrane, or surgical intervention (for pathologies of the nasal septum).
For vascular diseases, patients are prescribed drugs with antihypertensive and nootropic effects. Treatment is also carried out using vasoactive drugs.
To relieve pain and other symptomatic and clinical manifestations of neuralgia, local anesthetic drugs are used in medical practice in combination with non-narcotic painkillers and vasodilators. Their use is determined by which part of the nasociliary nerve has undergone pathological effects:
- For neuralgia of the long ciliary nerve, dicaine (0.25% eye solution) mixed with adrenaline (4 drops per 10 ml of solution) is used.
- For neuralgia of the subtrochlear region of the nasociliary nerve, cocaine hydrochloride (2% solution) mixed with 0.1% adrenaline (4 drops per 5 ml of the drug) is used. A solution of lidocaine is also used in the form of a spray, which is injected into the nasal passages on the corresponding side or both at once (for bilateral neuralgia) up to 4 times a day.
- If any part of the nerve is damaged, use a powder mixture of papaverine, glucose, antispasmodic, diphenhydramine and aminazine 2 times a day.
As an additional therapy for patients diagnosed with Charlin's syndrome, a course of intramuscular vitamin B12 is indicated, as well as intravenous administration of sulfonamide drugs.
Unpleasant chest pain is often a sign of heart disease. But a similar symptom is also characteristic of neuralgia. A competent specialist should be able to distinguish the signs of neuralgia in the heart area from heart pain. Read the article about how to treat this disease.
We will consider the causes and symptoms of trigeminal neuralgia in this topic.
Nerve damage during implantation: diagnosis, treatment, prevention
Damage to branches of the trigeminal nerve (eg, inferior alveolar, lingual, mental, or infraorbital) is a potential complication that can develop during the dental implant procedure.
Direct damage to the nerve fiber can be caused by injury, inflammation, or the result of an infectious factor. Most often, the branches of the trigeminal nerve are affected during anesthesia, flap separation, bone augmentation, osteotomy and direct installation of a titanium intraosseous support. Since the restoration of damaged nerve fibers is quite problematic, the best tactics for treating such complications is prevention. Therefore, it is extremely important for the doctor to understand the features of the histology and anatomy of the nerves of the maxillofacial region, and to be informed about the symptoms that most often accompany their lesions. Also, the clinician must take into account aspects of differential diagnosis in order to correctly establish the cause of the development of certain symptoms, based on which in the future he will have to carry out appropriate treatment. Treatment options for trigeminal nerve branch lesions include the use of various pharmacological agents, monitoring with physical therapy, or even removal of the problematic dental implant.
In this article we will discuss approaches to the treatment of dental patients with damage to the nerves of the maxillofacial area associated with the dental implantation procedure, as well as the main aspects of the etiology and pathogenesis of such pathologies in general.
Anatomy and histology of the trigeminal nerve
The trigeminal nerve is the fifth and largest pair of cranial nerves, which consists of the following branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). The mandibular nerve is the largest branch and innervates the lower lip, chin area, teeth, adjacent soft tissues, lower jaw and part of the external ear. The motor fibers of the mandibular nerve are not damaged during the implantation procedure because they arise from the main branch of V3 before exiting the mental foramen. The main structural unit of a nerve is the nerve fiber. The structure of V3 is dominated by myelinated nerve fibers. Each axon and Schwann cell is covered in connective tissue called the endoneurium. Groups of nerve fibers form bundles that are surrounded by epineurium. Damage to any part of the nerve bundle can lead to neurosensory impairment. The trigeminal nerve consists of 7000-12000 axons, and the number of bundles varies in different parts of the maxillofacial region. The inferior alveolar nerve (IAN) is polyfascicular (consisting of more than 10 fascicles), while the lingual nerve contains only a few similar nerve structures. Since the NAN is characterized by a large number of nerve bundles, its regenerative abilities are also significantly higher compared to the lingual nerve.
Types of nerve lesions
Lesions of the trigeminal nerve can be caused by compression, stretching, complete or partial disruption of the integrity of the nerve fiber. Damage may result in neurosensory changes in touch, pressure, temperature, and pain. Such pathologies significantly affect the patient’s comfort and ability to talk, eat, kiss, shave, apply makeup, brush teeth and drink normally. In addition, neurosensory disorders also affect the patient's ability to interact normally in society. Signs of these pathologies can be identified directly during surgery (if there is a pain symptom), or during long-term monitoring of the patient’s condition. The following terms are used to describe traumatic lesions of axons of varying degrees of complexity:
- neurapraxia - a lesion in which the integrity of the nerve fiber is preserved, and the mechanism of injury is associated with stretching or impact such as blunt trauma; Sensitivity usually returns to normal within a few days or weeks.
- axonotmesis - damage to the nerve, in which the processes of its degeneration and regeneration develop, but the axon itself does not lose its integrity, and sensitivity is normalized over 2-4 months; however, sensitivity after recovery may be slightly less than before the intervention, and in some clinical cases it is characterized by accompanying dysesthesia.
- neurotmesis - damage to the nerve, in which there is a violation of its integrity, and the prognosis for the restoration of normal sensitivity is unfavorable.
The International Association for the Study of Pain has standardized nomenclature regarding traumatic nerve injuries. In particular, the definition of the term paresthesia, which was previously used to refer to loss of sensation, was changed. Current terminology provides the following definitions:
- paresthesia - a change in sensitivity without accompanying discomfort;
- dysesthesia - a change in sensitivity, which is accompanied by unpleasant sensations;
- anesthesia - loss of sensitivity.
To describe changes in neurosensory functions, terms such as allodynia (the occurrence of pain to stimuli that normally do not provoke pain), causalgia (the presence of persistent burning pain), hypoesthesia (decreased sensitivity to the action of stimuli), hyperesthesia (increased sensitivity to action) are also used. irritants).
When nerves are stretched or compressed, the perineurium protects the bundles from damage. However, lengthening the nerve by more than 30% can provoke structural damage. When the integrity of the nerve is completely disrupted, symptoms of anesthesia and a decrease in certain sensory functions develop. When the integrity of the nerve fiber is partially disrupted, various symptoms of damage, including dysesthesia, may be observed. It should be noted that the presence of persistent pain after surgery is not a criterion for determining the potential for complete restoration of the function of the affected fiber.
After damage to the peripheral nerve, Wallerian degeneration begins to develop, which continues for several weeks and even months. Axonal necrosis develops distal to the site of traumatic intersection. Degeneration in such cases is progressive and irreversible and lasts for up to 18 months. The ability of the affected nerve area to heal is influenced by factors such as the patient's general health, age, and type of injury. A key point in the process of nerve recovery after damage is the formation of scar tissue in the area of endoneurial tubules.
Evaluation of traumatic lesions of the trigeminal nerve
NAN is most often affected during the installation of dental implants. Signs of inferior alveolar nerve involvement include anesthesia, paresthesia, or dysesthesia in the skin, lower lip, cheek, and gums up to the second molar site. Patients with damage to the lingual nerve are characterized by uncontrolled salivation, biting the tongue, a feeling of heartburn, loss of taste, changes in speech and swallowing function, numbness of the mucous membrane and tongue. Both during and after surgery, all potential symptoms of sensorineural impairment should be documented. Areas of altered sensitivity are mapped (both by location and area of the affected area). Thus, it is possible to monitor changes in all parameters in the future, and determine whether the patient needs microsurgical intervention or not. To identify and determine the extent of disorders, both objective and subjective diagnostic tests are used, which are conventionally divided into mechanoceptive (response to mechanical stimuli and compression) and nociceptive (sensation of pain).
Mechanoceptive tests include static touch with a soft brush, two-point discrimination, and determination of the direction of brush movement. The sensation of a needle prick and the recognition of thermal stimuli are classified as nociceptive diagnostic procedures. To compare indicators, not only the affected area is always diagnosed, but also a symmetrical area, thus accurately identifying the fact and degree of neurosensory impairment. If the patient complains of loss of taste, a cotton swab moistened with salt or sugar is used for diagnosis.
Prevalence of traumatic nerve injuries
After implantation, permanent loss of sensitivity in the lip area due to traumatic damage to nerve fibers is observed in 0-36% of clinical cases. However, these data can be considered somewhat outdated and do not correspond to the approaches of modern implantological practice. After all, earlier during operations, dental surgeons more often used vestibular incisions, which caused sensitivity disorders to develop. Today, during the installation of dental implants, midline mucosal incisions are made along the top of the residual ridge, and the entire procedure is pre-planned, taking into account the data obtained after a computed tomographic examination. Thus, it can be assumed that the prevalence of nerve fiber damage due to implantation is significantly less than 36%.
Dannan et al reported that the incidence of nerve damage with implantation was as high as 2.95% (5 of 169 patients treated) in cases of temporary neurosensory changes, and 1.7% in cases of irreversible implant-associated neuropathies. Another study found that the incidence of nerve damage after maxillofacial surgery was 2.69% (42 of 1559 patients), with an even lower percentage of irreversible neurosensory damage, but the exact number was not specified in the study. . In the author's opinion, however, even such rates of implant-associated damage to neural structures are too high for clinical practice. Transient loss of sensation in the lip can often be associated with swelling, which is observed during the first two weeks after surgery.
Traumatic damage to the lingual nerve during surgical procedures
The lingual nerve in the region of the mandibular molars passes through the soft tissues on the lingual side of the jaw. Sometimes the nerve is located coronal to the surface of the bone tissue and is tightly adjacent to the cortical bone plate on the lingual side. Therefore, any surgical interventions must be carried out very carefully in this area. After removal of the third molars of the mandible, lesions of the lingual nerve are observed in 0.5-2.1% of clinical cases. Traumatic disorders of the lingual nerve during dental implantation are not a common phenomenon and are recorded quite rarely. To prevent such complications when installing dental implants, the following rules should be followed: only intrasulcular incisions can be made without releasing incisions and flap separation from the lingual side; During flap separation, it is necessary to avoid overstretching it and maintain a safe distance when performing osteotomy. 90% of all recorded cases of neurosensory changes associated with lesions of the lingual nerve resolve within 8-10 weeks after surgery.
Preoperative planning: prevention of traumatic nerve injuries
To prevent most complications associated with the installation of dental implants, it is necessary to ensure careful planning of the surgical intervention. Using the capabilities of computed tomography and surgical templates allows you to avoid unexpected outcomes of iatrogenic intervention. When installing a dental implant, a minimum of 2 mm of bone thickness must be left between the apical part and the coronal part of the mandibular nerve canal. In addition, it is important to adhere to the specified osteotomy length and strictly follow the bone preparation protocol. The presence of 2 mm of bone thickness also avoids excessive bone compression in the area of the nerve after installation of a titanium intraosseous support (photos 1 - 2).
Photo 1. The implant was installed in the area of the 30th tooth. After the anesthesia wore off, the patient began to complain of parasthesia in the area of the right lip and chin. On an x-ray taken immediately after implantation, there are no signs of implant penetration into the mandibular nerve canal.
Photo 2. The implant was installed 10 years ago, and during this time the patient was able to adapt to changes in sensitivity. The CBCT image shows that the implant in the area of the 30th tooth is much closer to the nerve canal than previously thought.
If necessary, to ensure the safety of the intervention, short dental implants can be used. It is also important for the doctor to be familiar with the absolute length of all drills that are used during the manipulation, since failure to take these parameters into account can provoke excessive deepening by more than 0.4-1.5 mm relative to the selected safe boundary. To control the deepening into the bone tissue, it is also recommended to use special stoppers. However, the physician must understand that neither the thickness nor the density of the bone tissue over the area of the nerve ensures the safety of its condition during the osteotomy procedure, therefore applying too much force and pressure during the preparation of the bone tissue is strictly prohibited. Finally, it should be noted that up to 50% of lawsuits related to nerve damage after implantation are caused by the lack of informed consent on the part of the patient, which the doctor must obtain before surgery. It is also a good idea to assess the patient’s neurosensory parameters before the intervention in order to compare them with the data that will be obtained after implantation.
Local anesthesia: a potential cause of nerve damage
Traumatic lesions of the mandibular and lingual nerves can occur during anesthesia due to needle trauma, hematoma, and exposure to the components of the anesthetic solution. The mechanisms of such lesions are still quite unexplored. One retrospective study estimated the incidence of nerve injury during anesthesia to be between 1/26,762 and 1/160,5716 cases, while Haas and Lennon predicted the incidence of such complications to be 1/785,000. Other data suggest that the prevalence of short-term transient lesions of the mandibular and lingual nerves as a result of anesthesia ranges from 0.15% to 0.54%. Whereas cases of the development of permanent changes in sensitivity of the same etiology are quite rare, with a prevalence of 0.0001-0.01%. After performing mandibular anesthesia, 3-7% of patients experience electrical sensations that resolve spontaneously over time. However, when the clinician notes that the patient has overreacted to the needle insertion, the needle may need to be withdrawn and repositioned slightly. Methods for the treatment or prevention of nervous complications associated with the anesthesia procedure have not yet been developed. Between 70% and 89% of anesthesia-related neurosensory lesions develop in the region of the lingual nerve. This trend can be explained by the fact that the lingual nerve consists of only a few bundles, while the lower alveolar nerve consists of a huge number of them, which, in turn, increases its potential for regeneration. From a geometric point of view, everything is explained much more simply: the size of the needle is on average 0.45 mm, while the diameter of the lingual nerve is 1.86 mm, and the diameter of the inferior alveolar nerve is 3 mm.
Anesthesia-associated nephropathy most often develops after anesthesia with 4% solutions of articaine or pilocarpine. Compared to lidocaine, pilocarpine and articaine cause 7.3 and 3.6 times more neurosensory impairment. Garisto et al reported that in 4 of 9 studies, the complication rate was higher with prilocaine or articaine 4% solutions than with lower concentration anesthetic injections. According to the authors, local anesthesia with these drugs should be avoided in order to reduce the incidence of associated neuropathies after iatrogenic interventions. However, according to Malamed, the cases in which Arcticaine has demonstrated a greater association with neuropathies than lidocaine are anecdotal, and do not have sufficient evidence-based argumentation. Similarly, in 2013, after an extensive review of the literature, Toma and colleagues concluded that studies suggesting extreme neurotoxicity of articaine were retrospective in design, the data presented were at high risk of bias, and the findings should not be regarded as sufficient evidence. . The authors concluded that direct trauma to the nerve fiber is the predominant cause of neurosensory impairment, and the latter has little to do with the chemical toxicity of the anesthetics used. In general, there is disagreement in the literature on this issue; Therefore, clinicians should make decisions regarding the use of higher concentrations of anesthetics based on the conditions of each individual clinical case, while interpreting prior information and taking into account the recommendations of the drug manufacturers.
Osteotomy procedure for implantation
The osteotomy procedure should be performed using well-sharpened drills and abundant irrigation. Hypothetically, overheating of the intervention area during osteotomy can provoke traumatic nerve damage. The extent of bone necrosis caused by overheating is directly proportional to the preparation temperature under which iatrogenic intervention was performed.
Eriksson and Albrektsson summarized that performing an osteotomy at 47°C for 1 minute may provoke subsequent bone resorption. In cases of progressive resorption, given the shift in the position of the mental foramen, a transcrete incision is contraindicated; instead, it should be shifted to the lingual side. When placing implants anterior to the mental foramen, careful analysis of CT images should be performed, which may help detect the presence of a loop of mental nerve.
Flap separation procedures
As a rule, flap separation does not provoke any neurosensory disturbances, however, the doctor should still pay great attention when performing this iatrogenic intervention in the chin area. The doctor must clearly understand where the mental nerve exits the mental foramen, so that when separating the flap it does not cause damage to the nerve fiber.
Tooth extraction
Before extracting molars and premolars in the lower jaw for subsequent installation of dental implants, the relationship between the position of their roots relative to the course of the inferior alveolar and mental nerves should be carefully studied. Care should also be taken to curettage the sockets after resection, since periapical lesions may often be close to neural structures (Figures 3–4).
Photo 3. The patient sought help for pain in the area of the 31st tooth. The X-ray image shows signs of acute apical periodontitis.
Photo 4. The results of CBCT diagnostics indicate that the pathological focus is located near the nerve canal. The tooth was carefully removed, followed by careful curettage of the defect area.
Pharmacological therapy of neuropathies associated with the installation of dental implants
There is no clear opinion regarding which drugs are best to use in diagnosing traumatic lesions of the nerves of the maxillofacial area. Some authors prefer corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs). It is worth remembering that the use of various pharmacological agents is relevant only if the integrity of the nerve fiber as a whole is preserved. In cases of sensory impairment after injection, the patient can be given dexamethasone 4 g/ml directly to the area of the injury, and after 3 days the dose of steroids can be increased. If a nerve is compressed or a fiber is injured during surgery, 1-2 ml of dexamethasone is administered intravenously, after which dexamethasone is prescribed orally for 6 days (4 mg, 2 tablets after meals for three days, then 1 tablet after meals for another three days). If the integrity of the nerve is compromised, steroids may be prescribed for a period of 5 to 7 days. Impaired sensitivity can be relieved by 800 mg of ibuprofen, which should be taken three times a day for 3 weeks. If neuropathy develops after removal of the implant, patients can also be prescribed 800 mg of ibuprofen three times a day and with the same frequency 500 mg of amoxicillin for 5-7 days. In parallel, 50 g of prednisolone is prescribed, reducing the dose by 10 mg every day (for 5 days). Post-traumatic neuropathy can be treated with low doses of antidepressants and antiepileptic drugs.
In combination with a pharmaceutical approach, Renton and Yilma recommend physical therapy interventions for patients with chronic neuropathy. The goal of such a treatment algorithm is to reduce the patient’s discomfort and help him get rid of pain. Bagheri and Meyer generally question the effectiveness of corticosteroids in the treatment of traumatic lesions of the inferior alveolar nerve, since the density of the bone tissue forming the nerve canal is so high that it is simply impossible for medications to reach their space. At this time, there are no results from clinical studies that confirm the feasibility and success of using corticosteroids or NSAIDs for traumatic neuropathies associated with the installation of dental implants.
When to refer a patient to a microsurgeon
Universal recommendations regarding when to refer patients with traumatic trigeminal neuropathies to a microsurgeon have not yet been developed. Some authors argue that the patient should be referred to a specialist immediately after noticing sensory disturbances after the implantation procedure. Other researchers recommend referral to a microsurgeon after 2, 3, 4, or even 6 months of monitoring if signs of traumatic neuropathy are observed during these months. Ziccardi and Zuniga recommend microsurgical intervention for up to 1 year after the implantation procedure, since the effectiveness of microsurgical correction noticeably decreases one year after the intervention. When determining the fact of traumatic neuropathy after implantation, the physician must determine whether the patient needs to be referred to a microsurgeon, or whether the situation can be improved with the use of various pharmacological drugs.
Sometimes explantation, or slight “unscrewing” of the implant from the bone, can help resolve the symptoms of traumatic sensory dysfunction. The results of individual studies indicate that the advisability of referral to a microsurgeon is justified only by stating the fact of a complete violation of the integrity of the nerve. When the doctor is sure that he did not violate the integrity of the bone canal of the nerve, then the disturbance in sensitivity can be caused by compression of the nerve fiber or an inflammatory effect. In such cases, a pharmacological approach to treatment can also help. Meanwhile, the doctor must understand that even if he did not perforate the main nerve canal, the nerve often has additional branches, the course of which is quite difficult to predict. The latter is possible only with a careful analysis of the data obtained after CBCT scanning, because two-dimensional radiographs provoke superimposition of individual anatomical structures, therefore, the prediction of possible traumatic nerve damage with their help is ineffective (photo 1-2).
In cases of discomfort after implant installation, if it can be confirmed that the intraosseous support is not located near the nerve canal, treatment algorithms are variable. Bagheri and Meyer suggest monitoring for 3 to 4 months to see if sensory improvements are observed before referring the patient to a microsurgeon. The authors also claim that if the position of the implant is too close to the nerve, the level of compression of the latter can be reduced by a slight reverse movement of the implant. Other researchers suggest explanting the intraosseous construct within 36 hours and immediately prescribing steroids if the patient exhibits signs of neurosensory impairment after surgery.
Khawaja and Renton described four clinical cases of the development of sensory symptoms after the installation of dental implants, in which the intraosseous structure was not located near the nerve. However, upon explantation, the symptoms of neurosensory disorders resolved quite quickly in two of the four patients. Interestingly, positive dynamics were observed in patients whose implants were removed within 18 and 36 hours, and residual symptoms were observed in patients whose explantation was performed 3 and 4 days after surgery.
Renton, Dawood and colleagues suggest that patients with such symptoms should be monitored for no more than 3 months, since after this period the risk of developing neural changes outweighs the potential success of microsurgery. Ziccardi and Steinberg in their review article recommend initial monitoring for 1 month, and if symptoms improve, continue to monitor the patient. If the symptoms do not improve or worsen, it is necessary to refer the patient to a microsurgeon. Research results indicate that patients operated on for neurosensory impairment 6-8 months after implantation have the same level of success as patients operated on in a shorter period after iatrogenic intervention. According to the authors, earlier intervention is possible and more effective, but there is no evidence to support this assumption. In addition, microsurgery is not indicated in cases of only minor neurosensory changes, since the risk from the intervention itself in such cases exceeds the predicted success rate. There is also a 12-week waiting period - on average, this is the amount of time a doctor needs to decide whether to refer a patient for microsurgical surgery.
It is logical that in cases where patients have acute pain, it is not necessary to wait 12 weeks. Before arguing for certain approaches to treatment, the doctor must objectively compare X-ray data and clinical symptoms, and then take into account all possible medico-legal aspects of the violations.
Surgical restoration of damaged trigeminal nerve
There are specific reasons that justify the need for microsurgical restoration of the damaged trigeminal nerve, and contractual factors that determine the prognosis of this manipulation. Ziccardi and Zuniga formulated the following list of reasons that justify referral of a patient to a microsurgeon: sensory impairment that persists for more than 3 months and interferes with the patient’s normal life activities, confirmation of the fact of nerve transection, lack of improvement in signs of hypoesthesia, development of pain after implantation. After microsurgical correction is performed, a number of factors influence the success of it: the waiting time after implantation, the type and volume of the lesion, the degree of vascularization of the affected area, the experience of the surgeon, the method of harvesting and preparing the graft, the presence of tension in the repair area, the age and general health of the patient.
In fact, microsurgical restoration of the branches of the lingual and inferior alveolar nerves is possible. However, the success rate of such manipulations is very variable - on average, researchers indicate only 50-59.4% effectiveness of such treatment, and in only two studies the results of the intervention reached levels of 81.7% and 63.1%. In all the studies conducted, the number of subjects is quite small, so it is methodologically impossible to compare the final results of such studies. However, 50-60% of patients after microsurgical correction show signs of improvement in neurological impairment. Ziccardi and Zuniga warn that patients with severe sensorineural impairment should be informed that full rehabilitation is virtually impossible. Also, many researchers indicate that the effectiveness of microsurgical procedures in cases of treatment of anesthesia, dysesthesia and spontaneous pain is exaggerated. To summarize, microsurgical correction can help individual patients, but the level of predictability of the results of such treatment is ambiguous. Thus, the best treatment for neurosensory disorders associated with implantation is their prevention.
Conclusion
A common dilemma in clinical practice is: if an implant is successfully osseointegrated but causes mild paresthesia without pain, what should be done with it? After all, explantation may not always help resolve symptoms, and retention of the implant in the bone with actual nerve damage can trigger the development of neuroma. The latter is formed as a result of excessive healing of the area of the damaged nerve and hyperplasia of adjacent tissues, and very often requires subsequent surgical removal. The decision to choose a possible treatment method should be made together with the patient after a thorough discussion of all possible options, and before starting rehabilitation, the patient must formally confirm his consent by filling out a special written form.
Authors: Gary Greenstein, DDS, MS Joseph R. Carpentieri, DDS John Cavallaro, DDS
Source of the article: https://stomatologclub.ru/stati/implantologiya-14/porazhenie-nerva-vo-vremya-implantacii-diagnostika-lechenie-profilaktika-2895/
Diagnosis of neuralgia
A neurologist is involved in differential diagnosis. Diagnosis of neuralgia begins with a neurological examination of a patient with typical complaints for this disease. The listed causes of neuralgia require a more complete examination to identify or exclude the underlying disease.
In some cases, additional instrumental examination (electroneurography) may be required if the cause of neuralgia was injury in the projection of the nerve. An MRI of the spine or any of the nerve plexuses may be required in the event of any volumetric impact on the nerve structures, as occurs with a herniated or protruded intervertebral disc or soft tissue tumors.