Giuseppe Cantatore University of Verona (Italy). Department of Endodontics. Assistant professor.
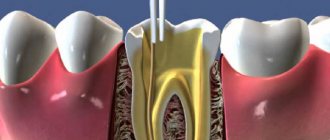
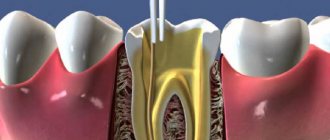
In a classic study published in 1985, Bystrom et al. compared the sterilizing effectiveness of three different methods of endodontic treatment of infected canals and found that mechanical treatment in combination with irrigation with saline solution ensures canal sterility in 20% of cases, while replacing NaCl with a 5% sodium hypochlorite solution leads to canal sterility in 50% of cases. cases, and the addition of the latter scheme with a single temporary filling of the canal with calcium hydroxide increases the percentage of canal sterilization to 97%. Does this mean that when treating infected root canals, temporary filling with medicated paste is required in all cases? More than 15 years have passed since the Bystrom et Al study; Today we know much more about the properties of microorganisms associated with pulp-periodontal pathology: from virulence to mobility, from the ability to penetrate dentinal tubules to sensitivity to various antiseptics. 15 years of research experience have shown that many irrigation solutions have pronounced bactericidal activity against microorganisms such as enterococcus faecalis or candida, which are resistant to calcium hydroxide or chlorophenol. In this article, we will discuss in 7 points how to improve the root canal irrigation procedure, using the right products in the right sequence, in order to reduce the need for additional medicinal treatment of the canals between visits.
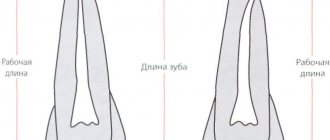
Key Factors for Effective Cleaning and Irrigation of the Root Canal System
1. Careful diagnosis of existing pulp-periodontal pathology
2. Taking into account the condition of the tooth tissues and the complexity of the anatomy of the root canal system 3. Removal of the endodontic smear layer 4. Compliance with indications when choosing means for irrigation 5. Optimization of the active components of the irrigation solution 6. Correct sequence of application of the irrigation solution during root canal treatment 7. Mandatory costs of at least 5 minutes for irrigation before filling
Adhesive systems
Dental composite materials do not have independent adhesion (a connection of a physical and chemical nature between dissimilar surfaces) to dental tissues. Therefore, filling teeth with composites requires the mandatory use of special adhesive systems (bonds). In other words, to create a strong connection between the composite and tooth tissues, it is necessary to use additional materials that have chemical or micromechanical adhesion to tooth tissues. Failure to comply with this condition leads to disruption of the adhesion of the composite to the dental tissues (due to shrinkage of the composite during polymerization) and the appearance of a marginal gap, the occurrence of secondary caries and sometimes damage to the pulp. The main components of the organic matrix of composites have fairly high adhesion to enamel, but in relation to wet dentin they behave as hydrophobic substances that do not adhere well to its surface.
Tooth enamel consists mainly of an inorganic substance (biological apatite, about 95% by weight), an organic component (collagen fibers, 1-1.5%) and water (4%). Thanks to this composition, the enamel can be dried, which ensures good adhesion of the hydrophobic organic component of the composite. To increase the effectiveness of adhesion of enamel and composite, the filling (restoration) technique involves preliminary acid etching of the enamel with a liquid or gel based on phosphoric (10-37%) or maleic (10%) acid. As a result of acid etching, organic plaque is removed from the enamel surface, proteins are denatured and, most importantly, enamel microporosity is formed due to the dissolution of areas of enamel prisms and substances in the interprismatic space to a depth of about 40 microns. After removing the etching agent with water and thoroughly drying, the surface is treated with enamel adhesives, which are a mixture of low-viscosity monomers (usually without filler), chemical composition close to the organic matrix of the composite and capable of penetrating into the spaces between the prisms of the etched enamel. Therefore, after polymerization, the enamel adhesive forms a mechanical bond with the enamel (due to polymerization in the micropores of the enamel) and a chemical bond (due to copolymerization) with the organic matrix of the filling composite.
Tooth dentin consists of inorganic substances (biological apatite, 70-72%), an organic component (collagen and other proteins, carbohydrates) and water (10%). Unlike enamel, dentin is penetrated by a large number of dentinal tubules filled with dentinal fluid, pulp substance, and cellular processes. The dentin surface is always moist, as fluid constantly flows through the dentinal tubules. Therefore, dentinal adhesion is a more complex problem, the modern solution of which takes into account a number of specific factors.
Since the dentin surface is always wet, dentin adhesive systems must contain hydrophilic components that can wet the dentin surface and penetrate the dentin tubules.
After removing tissues affected by caries, a “dentinal wound” is formed (exposure of dentinal tubules, damage to odontoblast processes, etc.), through which toxins and chemical reagents can penetrate into the dental pulp. Therefore, measures aimed at sealing the dentin surface are necessary.
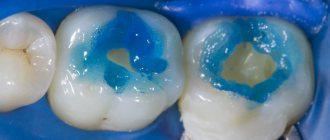
As a result of instrumental processing of dentin, so-called dentin is formed on its surface. smear layer (an amorphous layer approximately 5 µm thick) consisting of inorganic particles, denatured collagen fibers, and destroyed remains of odontoblasts. This layer makes it difficult for adhesive systems to diffuse into the surface layers of dentin. Preliminary acid etching of the dentin surface improves adhesion to the dentin adhesive due to the opening of the dentin tubules, demineralization of the surface layer and (for example, when using 35-37% phosphoric acid) removal of the smear layer. Etching does not have a harmful effect on the dental pulp.
- Methods for using dentin adhesive systems include the following main steps.
- Cleaning (conditioning, etching) of prepared dentin with acid solutions (phosphoric, citric, etc.), chelating reagents, and ethylenediaminetetraacetic acid (EDTA) solution.
- The use of a primer (primer), which is a solution of acidic and hydrophilic polymerizable monomers. The need to use primers is mainly due to the presence of dentinal tubules.
- The use of dentin adhesive (a chemical compound that ensures the formation of a bond between the filling composite and the primer layer on the dentin surface).
Despite differences in application techniques and compositions, modern dentin adhesive systems are united by the fact that they are all based on solutions of hydrophilic methacrylates. Along with their high activity towards the collagen structure of dentin, they easily polymerize in the hydrophilic environment of dental tissue. The first 2 stages are preparatory and facilitate the penetration of dentinal adhesives (due to the hydrophilic parts) into the dentinal tubules and spaces previously occupied by biological apatite, followed by encapsulation of collagen fibers. After polymerization of the adhesive, a thin layer of substance is formed, consisting of adhesive components and collagen fibers of dentin (the so-called hybrid layer). The hybrid layer ensures reliable fixation of the composite to the dentin (during subsequent filling of the cavity) and is a protective barrier against the penetration of microorganisms and chemicals into the dentinal tubules and tooth cavity, blocks the movement of fluid in the dentinal tubules and prevents postoperative sensitivity. The adhesion strength of the hybrid layer to the dentin surface is very high and exceeds the adhesion strength of natural dentin and enamel. Typically, modern dentin adhesive systems contain components that combine Stages 1 and 2 (self-conditioning, self-etching primers), Stages 2 and 3 (single-pack formulations), or Stages 1, 2 and 3 (single-stage, single-stage preparations, self-etching adhesives)
Currently, there is a wide variety of dentin adhesive systems, each of which has a unique chemical composition and application characteristics. But the mechanisms of their adhesion to dentin can be classified in relation to their effect on the smear layer (i.e., according to the different way in which the hybrid layer is formed).
- The adhesive system preserves and modifies (strengthens) the smear layer due to its impregnation with hydrophilic low-viscosity monomers followed by polymerization. Adhesion in this case occurs due to the connection of the modified smear layer with the structural elements of dentin and, on the other hand, due to the chemical connection with the filling composite. The disadvantage of these systems is shallow penetration into the smear layer and insufficient adhesion.
- The adhesive system transforms the smear layer through the action of a self-conditioning primer, which includes hydrophilic monomers and organic acids (for example, maleic). When exposed to such a primer, the smear layer is partially dissolved, the dentin surface is demineralized and the dentinal tubules partially open (into which hydrophilic components of the adhesive can enter to form polymer processes). In addition, reactive groups of adhesive molecules (amine, carboxyl, etc.) can interact with functional groups of molecules of organic components of dentin. In this case, the hybrid layer has a more complex structure.
- Dissolution and removal of the smear layer as a result of acid etching of the dentin surface followed by rinsing. At the same time, the dentinal tubules open, the dentin surface is demineralized, and collagen fibers are exposed. The components of the adhesive system penetrate the dentinal tubules and the demineralized surface layer of dentin and bind collagen fibers. This hybrid layer provides a strong bond with dental tissues.
In modern dentistry, adhesive systems of several generations are used.
4th generation adhesive systems contain 3 components: an etchant or conditioner (for etching enamel and dentin), a primer (a mixture of hydrophilic monomers) and an adhesive. A three-stage technique is provided - etching (enamel takes a longer time than dentin) followed by washing and drying, application of a primer with drying (primer contact with the enamel does not affect the adhesion strength; when etching only enamel, the use of a primer is not necessary), application and polymerization of the adhesive. They provide an adhesion force to enamel and dentin of about 30 MPa.
5th generation adhesive systems are preparations in which the primer and adhesive are combined (one-component system). They provide a two-stage technique - etching (conditioning) and application of a one-component adhesive. These adhesive systems are easier to use, but the adhesion strength is slightly less (10-30% in laboratory conditions) than that of the 4th generation adhesive systems.
6th and 7th generation adhesive systems are one-step preparations that combine the properties of a cleaner (conditioner, etching agent), primer and adhesive. Not yet widely used.
Adhesive systems also differ in the technique of etching dental tissue. The selective etching technique involves separately etching dentin and enamel (usually with different etching agents). Complete (total) etching is carried out with the same etching agent applied to both enamel and dentin. Conditioners (contain low concentration acids) have appeared as part of modern adhesive systems. The mechanism of action of conditioners is identical to the mechanism of action of etching agents (containing acids in higher concentrations). Conditioners are less aggressive and penetrate to a lesser depth into the enamel and dentin; they are used when the resistance of dental tissues to caries is low.
Adhesive systems are cured in three ways:
- under the influence of light (light-curing) (used in the frontal group of teeth, where light access can be easily obtained);
- chemical and light (double curing) (consist of a base and a catalyst, after mixing which light curing is carried out followed by final chemical curing; used for chewing teeth);
- chemically (self-curing) (used in cases where light exposure is difficult).
When using adhesive systems, lining materials are used only in deep carious cavities (pointwise, within the peripulpal dentin). The treatment pad (based on calcium hydroxide) must be covered with an insulating pad, since the components of the treatment pad disrupt the polymerization process.
The fundamental issue with regard to adhesive systems is the purpose of using dentin adhesives, namely, strong fixation of a filling (restoration) or sealing the border of a filling with tooth tissue. Recent studies show that the fixation of the filling is ensured mainly by micromechanical adhesion to dentin, as well as adhesion of the composite to the enamel. This point of view assigns a secondary role to dentinal adhesion (the significance of dentinal adhesion is questioned), and the main thing is to ensure the tightness of the filling-dentin interface, prevent microleakage, and protect dentin and pulp.
Typical representatives of adhesive systems and their components are the following.
Etch-Rite>, Pulpdent. Etching gel for dentin and enamel. Contains phosphoric acid (38%), amorphous silica gel.
Gel Etchant. Etching agent, contains phosphoric acid (37.5%).
Prime Bond NT. Universal (for enamel and dentin), one-component (primer and adhesive together in one bottle) light-curing adhesive system. Contains nanofiller for increased strength and improved edge adaptation. When additionally mixed with a chemical polymerization activator (Self-Cure Activator), a dual-cure adhesive system is obtained (used for areas not easily accessible to light). The advantage of this system is the presence in its composition of a combination of 3 elastomers (polymers capable of large reversible deformations). This allows the adhesive layer to stretch when the filling (restoration) deforms under repeated chewing load and prevents depressurization of the connection between the composite and dental tissues.
Optibond Solo. A universal one-component light-curing system, barium glass is used as a filler component (25%).
These adhesive systems provide high adhesion strength of composites and compomers not only to dental tissues, but also to metals and ceramics.
Make an appointment with the best dentists in Moscow!
Pharmacodynamics and pharmacokinetics
First of all, you need to find out: EDTA, what is it? EDTA (ethylenediaminetetraacetate) is the name of the salts that the organic substance ethylenediaminetetraacetic acid . EDTA formula, using the example of disodium salt C10H14O8N2Na2•2H2O, sodium ethylenediaminetetraacetate C10H12CaN2O8•2Na. In the chemistry literature, the abbreviation EDTA is common, which is also applied to ethylene-diaminetetraacetic acid itself, but for the sake of distinction the term “acid form of EDTA” is used.
Pharmacodynamics
Salts of this acid are used in medicine for binding (chelation) and removing radioactive and toxic metals from the body. Cobalt salts of this substance are used as an antidote for hydrocyanic acid poisoning. Calcium ions are bound by EDTA disodium salt (known as Trilon B), lead, cadmium, cobalt, cerium, mercury, uranium ions - the drug Tetacin-calcium , which is a sodium-calcium salt of EDTA. It does not penetrate red blood cells, but removes metals from the extracellular space.
By removing heavy metals, it acts as an antioxidant , because heavy metals promote the formation of free oxygen radicals. In addition, heavy metals suppress the action of enzymes ( peroxidase , catalase ) that neutralize free radicals, disrupt the function of structural proteins, and when combined with DNA, they contribute to the appearance of numerous mutations.
Removes calcium from cholesterol plaques in blood vessels. Plaques decrease in size, small plaques disappear, arteries become elastic, the rheological properties of the blood improve, and cholesterol decreases. The dissolution and removal of calcifications is noted in the inner ear, in the kidneys, and joint mobility improves. As an anticoagulant, it reduces blood clotting.
In addition, it is used for blood preservation, as an anticoagulant in the separation of blood plasma in the production of drugs, and as a stabilizer of adenosine triphosphate in determining blood glucose. As a substance used in medicine, it has undergone serious laboratory studies that have confirmed its safety. EDTA has very low toxicity. It is not absorbed by the human body.
Pharmacokinetics
Does not disintegrate in the body. Less than 5% enters the cerebrospinal fluid and is quickly excreted by the kidneys. T1/2 is 20-60 minutes.
Blood with EDTA, what is it?
For the collection and transportation of capillary blood, there are special microvette tubes - modern systems in which the potassium salt of ethylenediaminetetraacetic acid is used as a filler.
Blood with EDTA does not clot, since the potassium salt of EDTA, applied to the walls of the test tube, is an anticoagulant, and evenly mixed with the sample, it prevents clotting. The micro-tube has a built-in capillary and is clearly marked and graduated. Microvettes 100 µl allow you to draw blood in a volume of 0.1 ml per hematological indicator.
Microvettes with EDTA 200 µl are designed for a volume of 0.2 ml and are recommended for collecting blood for clinical analysis (expanded). Microvette 500 - for a blood volume of 0.5 ml. Working with such systems is simple and convenient. The parts are made of clear, unbreakable plastic.
Indications for use
- heavy metal poisoning;
- angina pectoris , atherosclerosis , arrhythmias , hypertension, circulatory disorders in the extremities;
- increased levels of cholesterol, triglycerides, C-reactive protein, glucose, blood clotting;
- diabetic neuropathy;
- chronic fatigue syndrome;
- insomnia , memory impairment, irritability, depression, age-related changes in intelligence;
- multiple sclerosis , and Alzheimer's disease ;
- bursitis , osteoarthritis ;
- cataract , glaucoma ;
- psoriasis;
- allergic diseases;
- frequent ARVI;
- impotence , prostatitis .
Reviews
Based on the results of these studies, it was concluded that the substance does not pose a threat to the environment and is safe for people and animals. The positive safety finding has expanded the use of this substance, as evidenced by the growth of the EDTA market in recent years.
In the experiments of T.L. Dubina, which were carried out in 1968-1975, the following conclusions were drawn: “... slows down the development of atherosclerosis, hypertension and hypercholesterolemia. Does not affect the aging process, but prolongs life by reducing morbidity."
reviews about chelation therapy with EDTA; most often these are reviews from foreigners, which are submitted in translation. Cheletotherapy is more common abroad - USA, Israel, Canada. Mexico. Reviews are varied.
“I use chelation and my lipids are normal.”
“...took 250 mg. My vision has improved with EDTA."
“My husband has diabetic neuropathy - after taking it, his blood circulation improved.”
“...I feel much better, less pain in joints and muscles.”
“It’s clear if used for heavy metal poisoning, and not for an experiment on a healthy organism.”
"There is a potential for significant impairment of kidney function as bound heavy metals pass through the kidneys."
Synthesis
The compound was first described in 1935 by Ferdinand Münz, who prepared the compound from ethylenediamine and chloroacetic acid. Today, EDTA is primarily synthesized from ethylenediamine (1,2-diaminoethane), formaldehyde, and sodium cyanide. This route yields tetrasodium EDTA, which is converted in a subsequent step to acidic forms:
H 2 NCH 2 CH 2 NH 2 + 4 CH 2 O + 4 NaCN + 4 H 2 O → (SAK 2 CCH 2 ) 2 NCH 2 CH 2 N (CH 2 CO 2 Na) 2 + 4 NH 3 (SAK 2 CCH 2) 2 NCH 2 CH 2 N (CH 2 CO 2 Na) 2 + 4 HCl → (HO 2 CCH 2) 2 NCH 2 CH 2 N (CH 2 CO 2 H) 2 + 4 NaCl
This process is used to produce approximately 80,000 tons of EDTA each year. Impurities produced by this route include glycine and nitrilotriacetic acid; they arise from ammonia coproduct reactions.
Formula, release form
The formula of EDTA is C10H16N2O8.
Main forms of release:
- Powder packaged in 10 grams.
- Solution in ampoules of 20 milliliters, 10 pieces per package.
- In capsules, 30 pieces per package. One capsule can have different dosages: 625, 440, 500 milligrams.
The cost of a pure product varies from 200 to 1000 rubles, depending on:
- cities of purchase;
- networks of pharmacies where acid is sold;
- manufacturer's name;
- forms of purchase and dosage.
EDTA is available without a prescription. The shelf life is 2 years from the date of manufacture. This date can be found on the top of the package.
This shelf life is observed if the drug was properly stored at a temperature not exceeding 25 degrees.
Replacement options
Similar in properties to E385 is disodium ethylenediaminetetraacetate (E386), which is also designated as EDTA. This substance is also considered an antioxidant, which has no analogues in nature, and belongs to the class of moderately dangerous.
Despite the harm caused by EDTA to human health and the environment, the substance is widely used in the chemical and food industries. E385 is added to:
- mayonnaise;
- juices;
- guilt;
- canned food;
- oils
People suffering from liver diseases, young children and allergy sufferers need to adhere to the permissible daily intake of no more than 2.5 mg/kg of weight or avoid such food altogether.
If you find an error, please select a piece of text and press Ctrl+Enter.
How do you know if a product contains calcium disodium salt?
Requirements for packaging of goods are specified in detail in Federal Law No. 29 “On the quality and safety of food products.” Manufacturers are required to indicate the names of all ingredients and their contents.
- The presence of calcium disodium salt is indicated by the alphanumeric code E385, or the abbreviation EDTA.
- If the product was imported from the EU or the USA, then the packaging must contain the designation E385, or one of the names - Komplexon II, EDTA.
- In Canada, the supplement may be called calcium disodium-EDTA.
- In the UK it is better known as Triplex II.