Anesthesia in dentistry
The best drugs for local anesthesia in dentistry
As we said, most often in dental clinics drugs based on articaine are used, in particular Ultracaine or Ubistezin. Now, taking into account the information presented, it is time to dwell in more detail on the advantages of such anesthetics.
Artikain
Modern local anesthesia in dentistry is unthinkable without articaine. It is this anesthetic that has a minimal ratio of toxicity and analgesic activity. In other words, among the most effective remedies, it is the least toxic.
Articaine is actively used for infiltration and conduction anesthesia. It is also used for intraosseous anesthesia before dental operations. But the surface anesthetic effect of articaine is not typical, which is why it is not suitable for applications.
The advantage of articaine is its high effectiveness against inflammation. (The inflammatory process reduces the effect of many drugs). This feature allows the use of Ultracain or Ubistezin in the treatment of purulent-inflammatory diseases of teeth and periodontium.
Due to its low toxicity, articaine is the best remedy for pediatric dentistry, the elderly, and patients with diseases of the hepatobiliary and urinary systems. Can be used while breastfeeding. Articaine practically does not penetrate into breast milk, and if it is found in it, it is in extremely low concentrations that have no clinical significance.
Can articaine be used during pregnancy? Studies have shown that although articaine crosses the placental barrier, it does not have a toxic or mutagenic effect on the fetus. Articaine can be used during pregnancy.
Lack of articaine in the average duration of action. It is quickly absorbed into the blood, quickly destroyed and excreted in the urine. This is partly due to the fact that articaine, like many local anesthetics, dilates blood vessels. For this reason, articaine is often used together with vasoconstrictors, which are already included in the drug. For example:
- Ultracain DS Forte, Ubistezin Forte contain adrenaline in a concentration of 1:100,000 (average concentration).
- Ultracain DS, Ubistezin contain adrenaline in a concentration of 1:200,000 (low concentration).
What if the patient belongs to a risk group and cannot be injected with an anesthetic with adrenaline? In this case, you need to use Ultracaine D - a drug with articaine without a vasoconstrictor component.
During pregnancy, it is recommended to use articaine in combination with adrenaline in low concentrations (Ubistezin, Ultracaine DS). Some experts believe that it is better not to use vasoconstrictors at all when treating pregnant women. Which option is better? The choice must be made taking into account individual characteristics. A woman should consult with specialists, including a gynecologist who monitors the pregnancy.
Lidocaine
Lidocaine is another popular and widely used drug, despite the fact that it is 2 times more toxic than procaine. The advantage of lidocaine is its average duration of action and more pronounced effect (it is 4 times stronger than procaine). The problem is that lidocaine dilates blood vessels, which requires the simultaneous use of high doses of a vasoconstrictor drug. For this reason, lidocaine is rarely used for conduction or infiltration, but is often used for superficial anesthesia.
Can lidocaine be used during pregnancy? We have already mentioned one drawback - the drug requires the use of adrenaline in high concentrations. The second disadvantage of lidocaine is that it is toxic, crosses the placenta and accumulates in the fetal liver. Considering the above, local anesthesia with lidocaine is contraindicated for pregnant women.
Mepivacaine
A unique drug, because it is the only anesthetic for local anesthesia with a vasoconstrictor effect. This feature allows the use of mepivacaine without a vasoconstrictor, which makes it the best tool for providing anesthetic benefits to patients at risk (diabetes, thyroid disease, hypertension and other cardiovascular diseases).
Mepivacaine is actively used for infiltration and conduction anesthesia. It is contraindicated during pregnancy because it penetrates the placental barrier and narrows the uterine arteries, which can lead to fetal hypoxia.
Anesthesia in pediatric dentistry
The problem of anesthesia in pediatric dentistry is quite acute, since it is psychologically and physically difficult for young patients to withstand a long treatment procedure. On the one hand, they are frightened by the noise of dental instruments, on the other hand, it is simply difficult for them to sit still for 40-60 minutes. In this regard, instead of local anesthesia, the dentist may recommend anesthesia.
Anesthesia in pediatric dentistry is carried out using a modern drug - Sevoran. Unlike the previously popular ZAX (nitrous oxide-oxygen mixture), Sevoran combines not only high efficiency (sufficient depth and duration of medicated sleep), but also maximum safety and a very “clean” recovery from anesthesia. After waking up, the child feels great; headaches, nausea or dizziness are extremely rare.
You can learn more about the methods of providing anesthesia in pediatric and adult dentistry at a consultation with a medical dentist.
Make an appointment
Intolerance to local anesthetics. Drug selection algorithm
The article discusses the problem of choosing a drug for local anesthesia from the point of view of preventing the development of adverse reactions associated with its clinical use, and provides the basic rules for selecting a drug in risk groups.
Table. Classification of local anesthetics
Introduction
Efficiency and safety are the main requirements for pharmacological drugs used for the treatment, prevention and diagnosis of diseases, as well as the main criteria for the successful use of drugs in clinical practice. Simultaneously with the increase in the number of drugs, the possibility of developing various undesirable reactions also increases. Adverse reactions that occur when using drugs occur in 1/10 of the world's population, but only 10% of them are caused by drug hypersensitivity [1]. These reactions may vary in clinical presentation, severity and have different outcomes.
As a rule, the pharmacological properties of therapeutic and diagnostic drugs are well known. However, a doctor cannot always predict hypersensitivity reactions [1].
An adverse reaction to a drug is an unexpected undesirable effect that occurs when using a drug in a therapeutic (preventive, diagnostic) dose.
Drug allergy is an increased sensitivity to a drug, which is based on immunological mechanisms.
Types of adverse reactions that occur when using drugs
There are two types of adverse reactions that occur when using pharmacological agents.
The first type of reactions are predictable side effects associated with the pharmacological action of drugs, depend on the dose used, can occur in any patient, and account for 75% of all adverse reactions to pharmacological agents. Pharmaceutical reference books and instructions for the use of drugs contain indications of the possibility of developing such reactions. The following are examples of predicted adverse reactions:
- toxicity of the drug;
- toxic reactions associated with overdose and/or accumulation of the drug;
- reactions caused by the pharmacological properties of drugs;
- reactions caused by the interaction of drugs;
- teratogenic effect of drugs;
- carcinogenic effect of drugs;
- mixed reactions [2].
Adverse reactions of the second type are unpredictable and are associated with reactivity and sometimes genetic characteristics of the patient. Let's name the options for unpredictable side reactions:
- Non-allergic congenital hypersensitivity (idiosyncrasy). Caused by enzyme deficiency.
- Drug hypersensitivity:
- allergic hypersensitivity (true allergic reactions: immunoglobulin E (IgE)-mediated and non-IgE-mediated). Immunological mechanisms are involved in these reactions;
- non-allergic hypersensitivity (pseudoallergy). It develops without the participation of immunological mechanisms [2].
Non-allergic congenital hypersensitivity (idiosyncrasy, fermentopathy) is a deficiency or incomplete activation of an enzyme involved in drug metabolism. Drugs or diagnostic agents can affect enzyme systems involved, for example, in the inactivation of serum kinins. If there is a deficiency of glucose-6-phosphate dehydrogenase, the use of oxidizing agents can lead to the development of hemolytic anemia in the patient. Sulfonamides, nitrofurans, and vitamin K preparations can also induce hemolysis in this group of patients.
Non-allergic hypersensitivity reactions, or pseudoallergic reactions, do not have immunological mechanisms, but can mimic the symptoms of an allergic reaction. The development of pseudoallergic reactions when using pharmacological agents may be associated with the direct or indirect release of histamine from mast cells and basophils, which occurs under the influence of the drug used. Some drugs are capable of non-immune activation of the complement system through the alternative pathway. As a result, activation intermediates with anaphylotoxin properties accumulate, which leads to increased membrane permeability, mast cell degranulation and histamine release. Pseudoallergic reactions may have other mechanisms. Non-steroidal anti-inflammatory drugs, salicylates, tartrazine can cause disruption of arachidonic acid metabolism. Accumulation of bradykinin may occur during treatment with angiotensin-converting enzyme inhibitors. When sulfites are used, sulfur oxide is formed, which has bronchoconstrictor properties [2].
Allergic reactions are distinguished by the participation of immunological mechanisms in their formation. Such reactions occur in a small number of patients, and their development is unpredictable. Being diagnosed with a drug allergy is a very serious decision. In case of erroneous denial of a drug allergy to a drug, its further use can lead to a tragic outcome. When drug allergies are overdiagnosed, the patient is deprived of the opportunity to use a whole group (sometimes several groups) of drugs that he sometimes needs [3, 4].
When using drugs, the development of psychophysiological adverse reactions is possible. They can occur in patients with hysterical neurosis, with autonomic disorders, as well as in patients who have suffered severe complications of drug therapy in the past.
Classification of local anesthetics
The emergence of a large number of modern, low-toxic local anesthetics (LA) has led to an expansion of their use in various fields of medicine and, unfortunately, to an increase in the number of patients who develop various adverse reactions when prescribed. Local anesthetics are drugs that reversibly inhibit the generation and conduction of impulses in peripheral nerves and nerve endings, which leads to loss of sensitivity in limited areas of the body [5, 6]. Types of local anesthesia vary depending on its clinical use in different areas of medicine:
- superficial anesthesia - application of MA to the skin, conjunctiva of the eye, mucous membrane of the nasal cavity, mouth, tracheobronchial tree, urinary tract, ear cavity;
- infiltration – dentistry, minor surgical interventions;
- conduction anesthesia - injection of a drug into the area of a nerve or plexus;
- epidural - injection of LA between the dura mater and the periosteum lining the inner surface of the spinal canal;
- spinal – injection of LA directly into the cerebrospinal fluid;
- intravenous regional anesthesia.
The use of this group of drugs is not limited to the field of medicine. In beauty salons, before painful procedures, MA is applied to the skin or injected. The patient may become sensitized to the drug and forget that he received procedures from a cosmetologist using MA. Most often, when interviewed, the patient does not mention such use of MA and almost never knows which drug was used. This is one of the reasons for various severity and sometimes fatal complications of various cosmetic procedures.
The chemical structure of LA determines their physicochemical characteristics and clinical properties. Local anesthetics consist of a hydrophobic (lipophilic) aromatic group, an intermediate ester or amide chain, and a hydrophilic secondary or tertiary amino group. Most MAs are tertiary ammonium bases, which are used in the form of solutions of water-soluble salts, mainly hydrochlorides. Basically, drugs differ in lipid solubility, binding to tissue proteins and dissociation constant [5, 6].
Depending on the intermediate chain, MAs are divided into esters and amides (table). The first group of local anesthetics (ester group) includes esters of benzoic acid (cocaine, Bencaine), esters of para-aminobenzoic acid (procaine, butamben), esters of aminobenzoic and benzoic acids (tetracaine). Ether MAs are relatively unstable in solutions and are quickly hydrolyzed in the body by pseudocholinesterase and some other esterases. One of the hydrolysis products is para-aminobenzoic acid, which can cause allergic reactions. The plasma half-life of these drugs is very short, so their tissue distribution is poorly understood.
The second group of MAs (group of amides) includes amides of heterocyclic and aromatic acids (Sovcaine, Novocaine), amides of aromatic amines - anilides (lidocaine, bupivacaine, mepivacaine, ropivacaine, etc.). Amide MAs are relatively stable in solution and are slowly hydrolyzed by liver microsomal enzymes. The rate of metabolism of different drugs is quite variable: prilocaine is the most rapidly metabolized drug, bupivacaine the slowest. In patients with impaired liver function, amide MAs may have a toxic effect due to prolongation of the drug's half-life; a decrease in hepatic blood flow leads to a slower inactivation of LA.
A small amount of drugs is excreted unchanged by the kidneys. A significant proportion of LA after administration enters the systemic circulation. The amount of absorbed drug and its peak plasma concentration depend on the dose and the presence of a vasoconstrictor in the solution (especially during conduction and infiltration anesthesia). The same dose of LA is associated with a different risk of toxic effects and depends on the anesthesia technique, the degree of tissue vascularization and interaction with tissue lipids. Anesthetics are actively absorbed through the mucous membranes. The binding of MA to plasma proteins affects pharmacokinetics and pharmacodynamics. Anesthetics of the ester group bind to plasma proteins slightly (
Cross-reactions of local anesthetics
Many drugs have similar chemical structures and have cross-allergenic properties. Among ether group MAs, cross-allergic reactions are quite common. A patient who is allergic to Novocaine should not use Anestezin, Dicaine. Cross-allergic reactions between drugs from the amide and anilide groups are quite rare. Research results (application tests) indicate the possibility of developing cross-allergic reactions between lidocaine, prilocaine, and mepivacaine. There are no cross-allergic reactions between drugs of the first (ether) and second (amide) groups. According to numerous data, the use of MAs from the group of amides and anilides leads to the development of adverse reactions, both allergic and non-allergic, much less often.
MAs of the ester group, being derivatives of benzoic, aminobenzoic and para-aminobenzoic acids, have common antigenic properties with drugs having a para-amino group and a benzenesulfonamide group [2, 4]. The para-amino group contains Novocaine and other benzoic acid esters; Menovazin, Anestezol, Almagel A and other combination drugs; Novocainamide; sulfonamides; para-aminobenzoic acid and its derivatives; para-aminosalicylic acid and its derivatives; ethacridine lactate. Sulfonamides have a benzenesulfonamide group; sulpiride; sulfonylurea derivatives (glycemic agents, sulfourea); diuretics containing a sulfamide group linked to a benzene ring; thiazide diuretics; carbonic anhydrase inhibitor; sotalol; para-aminobenzoic acid derivatives; para-aminosalicylic acid. If you are allergic to drugs of these groups, you cannot use MA of the ester group.
The patient may become sensitized when using medications that contain LA. Thus, benzocaine (Anestezin) is included in the preparations Almagel A, Anbital, Bellastesin, Pavestezin, Menovasin, and Anestezol suppositories. Lidocaine is contained in the preparations Aurobin, Lidactone, Strepsils Plus, Procto-Glivenol suppositories, Lidochlor (gel for topical use, contains lidocaine hydrochloride and chlorhexidine gluconate).
Procaine (Novocaine) is included in Solutan, Hemoroid ointments and suppositories, Sulfocamphocaine (a complex compound of sulfocamphoric acid and Novocaine). Trimecaine is contained in ointments Dioksikol, Dioksizol, Levosin.
In practice, cross-reactivity of pharmacological agents is not always taken into account. We must not forget that complex preparations may contain a substance to which the patient is sensitized.
Side effects of local anesthetics
Local anesthetics can cause toxic, allergic and pseudoallergic reactions. Most of the side effects that occur when using MA are associated with vegetative-vascular disorders, fear of surgery or dental procedures, toxic, hysterical reactions. Sometimes vasoconstrictors included in some local anesthetics have side effects. True allergic reactions to LA occur rarely [7, 8].
Toxic reactions
Local anesthetics and their metabolites are weak bases and easily cross the blood-brain barrier. The toxic effect is manifested by effects on the central nervous system (CNS), peripheral nervous system, cardiovascular system, and blood [5, 6, 7]. These reactions most often occur with an absolute overdose, when the dose of the drug is exceeded, or with a relative overdose associated with impaired drug metabolism. MAs have a “narrow therapeutic corridor,” that is, the interval between the maximum therapeutic and minimum toxic dose is small. Toxic reactions can also develop in diseases of the liver and kidneys.
The intensity of toxic reactions is divided according to severity [6]. Mild toxic reactions are manifested by depression of the central nervous system, lethargy, dizziness, drowsiness, nausea, tachycardia, and fluctuations in blood pressure. Severe toxic reactions are manifested by increased reflex excitability, motor restlessness, vomiting, the appearance of nystagmus, tremors, visual and auditory disturbances, severe arterial hypotension, bradycardia, and bradyarrhythmia. In severe toxic reactions, heart rhythm disturbances, angina attacks, tonic, clonic convulsions, paralysis of the respiratory and vasomotor centers, and cardiac arrest are possible.
MAs suppress cortical inhibitory pathways, resulting in uncontrolled activity of the excitatory component. The stage of unbalanced excitation with a further increase in the level of the drug can turn into generalized inhibition of the central nervous system. When applied in too large doses, LA can have a toxic effect on nerve tissue. The effect of MA on the cardiovascular system is associated with both a direct effect on the myocardium and an indirect effect on autonomic nerve endings. Typically, cardiovascular complications develop after the onset of neurological symptoms. All MAs, except cocaine, reduce the force of heart contractions and cause vasodilation, which leads to arterial hypotension. Collapse often develops when high doses of anesthetic are used, but sometimes when relatively small doses of drugs for infiltration anesthesia are used.
It is known that the administration of large doses of prilocaine can lead to the accumulation of a metabolite that is capable of converting hemoglobin into methemoglobin. High levels of methemoglobin are tolerable for healthy people, but can cause decompensation in patients with heart and lung diseases [7, 8, 9].
Non-allergic hypersensitivity (enzymopathy)
A lack of enzymes involved in the metabolism of drugs, or their incomplete activation, can lead to the development of adverse reactions. Insufficient pseudocholinesterase activity may impair the metabolism of essential MAs.
Allergic reactions
True allergic reactions to MA, in the formation of which immunological mechanisms take part, develop quite rarely and account for about 1% of all adverse reactions to this group of drugs. Immediate reactions, IgE-mediated, can manifest as urticaria, angioedema, symptoms of allergic rhinitis, conjunctivitis, anaphylaxis reactions, the severe degree of which is anaphylactic shock. Contact reactions at the site of LA application (swelling, hyperemia) are more common. There are known cases of delayed reactions that can occur several hours after administration of the drug and manifest themselves in the form of various exanthems, erythroderma, erythema nodosum, and in rare cases, allergic vasculitis [10, 11, 12].
Pseudoallergic reactions
Non-allergic hypersensitivity reactions are characterized by the fact that they are mediated by the same mediators as true allergies; clinical manifestations imitate allergies, but immunological mechanisms are not involved in their formation. The mechanism of such reactions may be associated with direct nonspecific release of histamine from basophils and mast cells or activation of the complement system through an alternative pathway. Clinically, pseudoallergic reactions can manifest themselves as various exanthemas, anaphylactoid shock, skin hyperemia, generalized itching, acute rhinitis, bronchospasm, and dysfunction of the gastrointestinal tract. The severity of such reactions depends on the rate of LA administration, its concentration, route of administration, as well as the content of mast cells at the site of drug administration. The development of true allergic reactions does not depend on these factors.
Groups at risk of adverse reactions to local anesthetics
There are groups of people whose risk of adverse reactions is increased. This applies to any adverse reactions to MA [12, 13]. The risk group for the development of adverse reactions to the use of MA includes:
- persons who have previously suffered reactions to MA;
- patients with an allergic disease (the presence of an allergic disease in a patient is a risk factor for the development of anaphylaxis);
- patients with coronary heart disease (CHD), unstable angina, receiving beta-blocker therapy;
- patients with intended administration of a large volume of LA;
- patients in whom MA is used frequently, at short intervals, which happens during oral sanitation, prosthetics, etc.;
- pregnant and lactating women;
- patients with a pronounced fear reaction before the procedure;
- elderly patients.
In elderly patients, the development of adverse reactions may be associated with a decrease in body size, water volume, and muscle mass. In such patients, the volume of distribution of drugs decreases. With age, the glomerular filtration rate and renal tubular function decrease (by approximately 30% by age 65%), blood flow in vital organs slows down, the binding capacity of blood plasma decreases, and the metabolizing function of the liver suffers. Concomitant diseases can lead to impaired metabolism and excretion of drugs. Hypoalbuminemia leads to a decrease in the binding of drugs to proteins and an increase in the free active fraction of the drug [14].
The role of vasoconstrictors, preservatives and stabilizers in the development of adverse reactions when using local anesthetics
Many LA cause vasodilation and are therefore rapidly absorbed from the injection site. To increase the potency and duration of action, vasoconstrictors are often added to MA, which reduce the systemic toxicity of anesthetics and increase their therapeutic index by slowing absorption. To block the conduction of impulses along nerve fibers, only one MA is sufficient, but to prolong its action and enhance the effect, vasoconstrictors such as Adrenaline (epinephrine), Noradrenaline (norepinephrine), Mesaton (phenylephrine), Octapressin (felypressin) are used. A vasoconstrictor is necessary to contract blood vessels, slow down the absorption of local anesthetic, and create a high concentration of the latter at the injection site. This enhances the effect of anesthesia and reduces toxic effects [5, 6, 7].
Adrenaline is the most powerful vasoconstrictor. It can have an undesirable effect on the adrenergic receptors of the heart (tachycardia), blood vessels (severe vasoconstriction), liver, cause an increase in blood sugar levels, and promote contraction of the uterine muscles. A possible increase in intraocular pressure in narrow-angle glaucoma and cardiac decompensation in patients with cardiovascular diseases can be dangerous. Some MA solutions used in dental practice contain the vasoconstrictor felypressin. This non-catecholamine vasoconstrictor in its chemical structure resembles vasopressin, a hormone of the posterior pituitary gland. Felypressin affects only peripheral blood vessels and has no effect on the heart. It can be used in patients with coronary artery disease and other diseases in which the use of catecholamine is contraindicated.
There are forms of local anesthetics that do not contain vasoconstrictors. Information about the content of the vasoconstrictor and its concentration is important when choosing a drug for anesthesia in risk groups.
Those at risk for developing adverse reactions to a vasoconstrictor include:
- patients with cardiovascular diseases (hypertension, coronary heart disease, heart failure);
- pregnant women (risk of uterine muscle spasms);
- elderly patients (comorbidities and basic therapy for many of them);
- patients receiving treatment with glucocorticosteroids (high doses, long-term use of which can affect the condition of the adrenal glands), tricyclic antidepressants, monoamine oxidase inhibitors, drugs with alpha-adrenergic blocking activity, rauwolfia drugs, thyroid-stimulating hormones (these individuals may have a high sensitivity to adrenaline) [15] .
The preservative (parahydroxybenzoate), which is part of MA, serves to increase the shelf life of drugs. The presence of this component in the drug also requires an analysis of its tolerability [6, 12, 13]. Parahydroxybenzoates are included in various cosmetics, creams, toothpastes and can cause contact allergic reactions. A related chemical compound - para-aminobenzoic acid - is a metabolite of Novocaine, that is, people who are allergic to essential MAs are likely to develop an allergic reaction to preservatives - parahydroxybenzoic acid esters. There is a danger of developing allergic reactions to preservatives in persons allergic to drugs containing a para-amino group and a benzenesulfonamide group.
Stabilizers (sodium or potassium disulfite) are added to solutions containing epinephrine to prevent oxidation and increase the stability of the vasoconstrictor [5, 7, 13]. They can cause undesirable reactions with hypersensitivity to sulfites and the development of bronchospasm in patients with bronchial asthma.
Thus, to make a decision on the use of a specific local anesthetic, information about the drug itself, the somatic status of the patient and all the drugs he uses, including topical ones, is necessary.
Principles for selecting a local anesthetic in risk groups
In patients with coronary artery disease, the use of catecholamines can increase myocardial hypoxia, therefore, MA is used without vasoconstrictors or in a minimal dose of the latter, the drug is administered slowly.
In patients with diabetes mellitus, MA without catecholamines is used or felypressin is used as a vasoconstrictor.
For kidney diseases, the least toxic MAs with rapid metabolism are recommended.
In case of liver diseases with impaired liver function, MAs that are metabolized in the liver should not be prescribed, since the toxicity of MAs increases due to decreased blood flow and hypoproteinemia. For such patients, ether anesthetics, which are not metabolized by the liver, are preferable.
For pregnant and lactating women, the least toxic MAs, short-acting, are used.
For glaucoma, MA without vasoconstrictors is used; Felypressin can be used as a vasoconstrictor.
In elderly patients, drugs without catecholamines or with minimal concentrations of catecholamines are used for local anesthesia [12, 13, 15].
Patients with allergic diseases are a special group of people. To decide on the use of MA in these patients, a detailed allergic and pharmacological history is especially important. Information about concomitant diseases and their basic therapy is required. The tolerability of both the MA itself and the vasoconstrictor, preservative and stabilizer contained in the solution should be taken into account. In patients with sulfonamide intolerance, ethereal MAs should not be used. For patients with bronchial asthma, it is dangerous to use MA containing stabilizers (sodium and potassium disulfites) due to the risk of developing bronchospasm. Information about previous reactions to drugs of other groups will help to avoid the development of cross allergic reactions. It is preferable (taking into account the medical history) to prescribe MAs of the amide group without stabilizers and preservatives. It is especially dangerous for such patients to exceed the dose! The patient must have a “Passport of a patient with an allergic disease”.
Prevention of adverse reactions to local anesthetics
1. It is necessary to compare the risk of possible complications with the negative consequences of refusing to use MA.
2. The tolerability of local anesthesia should be clarified if it has been performed previously.
3. If an allergic reaction to MA has been observed in the past, a drug belonging to a different group should be used. Thus, if the “suspected” drug is an ester derivative, drugs of the amide group can be used. In case of a reaction to MA containing an amide group, it is possible to use another amide compound (the possibility of cross-reactions between lidocaine, prilocaine and mepivacaine is assumed) [13].
4. In case of severe previous reactions (both allergic and non-allergic), it is necessary to stop using MA.
5. It is necessary to determine the degree of risk not only of the surgical intervention itself, but also of the use of local anesthesia.
6. Do not use drugs whose tolerability is questionable.
7. Premedication according to indications.
Premedication
Before surgery, in patients with allergic diseases in the acute phase, antihistamines (chloropyramine or clemastine) and glucocorticosteroids (prednisolone, dexamethasone) are administered parenterally. The dose of drugs and the frequency of their administration depend on the patient’s condition, concomitant diseases and the basic therapy used by the patient [4].
Persons with pronounced fear reactions (these persons are at risk) are recommended to take sedatives before manipulation [13].
When is it necessary to perform diagnostic tests with local anesthetics?
It is not always possible to find out which particular MA previously caused the reaction. In some cases, it is not possible to determine the nature of the reaction (allergic, pseudoallergic, toxic) only on the basis of anamnesis. Unfortunately, there are still no laboratory tests whose results can be trusted 100%. In some cases, specific IgE antibodies to certain MAs are determined. But these tests are informative only for immediate allergic reactions, IgE-mediated. The presence of specific Igs belonging to other classes weakly correlates with clinical manifestations and is not considered a reliable sign of allergy. The State Scientific Center “Institute of Immunology of the Federal Medical and Biological Agency of Russia” has developed and successfully used a test for inhibition of natural leukocyte emigration in vivo according to A.D. Ado, including with MA. Laboratory tests that are widely used today give a high percentage of false positive results. Some techniques are labor-intensive, expensive, and require a well-equipped immunological laboratory, which makes them difficult to use for routine examination. There are no reliable tests that could identify the possibility of developing a pseudo-allergic reaction. In rare cases, it is necessary to select a specific drug, namely if:
- Previously, when using MA, the patient experienced any side effects, the mechanism of which is not clear;
- there are no medical documents describing the clinical symptoms of reactions that occurred previously;
- the measures that were taken to stop the reaction that occurred are not known;
- There is no information about the drugs used for anesthesia and whether the patient received any concomitant therapy for the treatment of other diseases during this period of time [3].
And a few more rules that need to be followed when selecting a drug for local anesthesia.
- Skin and provocative tests with MA are carried out only according to strict indications, but are not used to satisfy the curiosity of the doctor or patient!
- No tests are performed on a drug that has previously caused reactions. For samples, MAs of another group are selected.
- Diagnostic tests are performed only by an allergist. The selection of the drug is carried out immediately before using MA. The examination is carried out in the treatment room. Must have an anti-shock kit!
- Before the drug selection procedure, the patient signs an informed consent.
- The patient should not use antihistamines at the time of examination (discontinue the drug 5–7 days before).
- A necessary condition is that MA should not contain a vasoconstrictor, preservative or stabilizer.
- The LA should not be cross-reactive with the drug that previously caused the reaction.
After maintaining 15-minute intervals, a prick test or a prick test with undiluted MA is performed (assessment of the reaction to test control and histamine is required). If a negative reaction is obtained, then 0.1 ml of the drug, diluted 1:100, is administered subcutaneously; if there is no reaction, the next dose of MA is 0.1 ml, diluted 1:10. Then 0.1 ml of undiluted drug is administered, and if there is no reaction, the patient receives a dose of 1.0 ml and 2.0 ml at fifteen-minute intervals [3].
The test results are assessed immediately; in the conclusion, it is clarified that currently no allergy to this drug has been identified; the total dose of LA administered to the patient is always indicated. The patient must remain under medical supervision for an hour [3].
There should not be a long time interval after selecting the drug until it is used. Knowing the peculiarities of the formation of drug allergies, one cannot exclude the possibility of sensitization over a long period of time.
Is there polyvalent intolerance to MA? It happens, but extremely rarely and most often of non-allergic origin. For such patients, they try not to use local anesthesia, and if necessary, general anesthesia is performed.
Conclusion
Thus, before performing a surgical or other intervention using a local anesthetic, the doctor must not only assess the scope of the operation and its risk for the patient, but also answer the questions:
1. Which MA can be used in this patient? It is necessary to take into account toxicity, duration of action, and the chemical group to which the drug belongs.
2. Is it possible to use MA with a vasoconstrictor? If possible, then with what vasoconstrictor (catecholamine or felypressin) and in what dose?
3. Does this MA contain preservatives and stabilizers and can they be used in this patient?
4. Does this patient need to consult an allergist? If consultation is necessary, what questions should he be asked?
5. Does the patient need premedication? What medications are needed and in what dose?
Very often, a careful analysis of the answers to the questions listed above helps to avoid complications.
Mechanisms of action and types of anesthetics
Anesthetics are divided into:
- Local
- General: a) inhalation - volatile liquids and gases b) non-inhalation (intravenous)
Table 1
1. Local anesthetics
Local anesthetics reversibly reduce the excitability of sensory nerve endings and block the conduction of afferent impulses in the nerve trunks in the area of direct application, and are used to eliminate pain.
The first drug of this group, cocaine, was isolated in 1860 by Albert Newman from the leaves of the South American shrub Erythroxylon coca. Newman, like many chemists of the past, tasted the new substance and noted a numbness in his tongue. Professor of the Military Medical Academy of St. Petersburg Vasily Konstantinovich Anrep in 1879. confirmed the ability of cocaine to cause anesthesia. In experiments on frogs, he discovered that cocaine had a “paralyzing effect” on the endings of sensory nerves. V.K. Anrep studied the effect of cocaine on himself: an injection of cocaine in a dose of 1 - 5 mg under the skin was accompanied by complete anesthesia - a pin prick or cauterization with a smoldering match did not cause pain. A similar effect was observed when a cocaine solution was instilled into the eye and applied to the mucous membrane of the tongue.
Local anesthetics are classified into esters (anesthesin, dicaine, novocaine) and substituted amides (lidocaine, trimecaine, bupivacaine). Local anesthetics - esters are hydrolyzed by blood pseudocholinesterase and act for 30 - 60 minutes. Their effect is prolonged by anticholinesterase drugs (prozerin). The hydrolysis product, n-amino-benzoic acid, weakens the bacteriostatic effect of sulfonamides. Substituted acid amides are inactivated by the liver monooxygenase system within 2-3 hours. Bupivaquine causes local anesthesia lasting 3-6 hours, after its cessation the analgesic effect persists for a long time.
From the point of view of practical use, anesthetics are divided into the following groups:
- Agents used for superficial (terminal) anesthesia: Cocaine, Dicaine, Anestezin, Pyromecaine
- Agents used primarily for infiltration and conduction anesthesia: Novocaine, Bupivacaine
- Agents used for all types of anesthesia: Lidocaine, Trimecaine
Mechanism of action
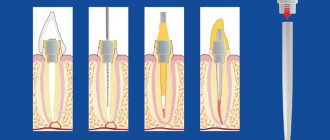
Local anesthetics are tertiary nitrogenous bases. They consist of hydrophilic and lipophilic parts connected by ester or amide bonds. The mechanism of action is determined by the lipophilic part, which has an aromatic structure. For application to mucous membranes and skin and parenteral administration, aqueous solutions of hydrochloride salts of local anesthetics are used. In the slightly alkaline environment of tissues (pH = 7.4), salts are hydrolyzed, releasing bases. The bases of local anesthetics dissolve in the lipids of the membranes of nerve endings and trunks, penetrate to the inner surface of the membrane, where they are converted into an ionized cationic form.
Figure 1 | Mechanism of action of local anesthetics
Receptors for local anesthetics are localized in the S6 segment of domain IV of the intracellular part of sodium channels. By binding to receptors, local anesthetic cations prolong the inactivated state of sodium channels, which delays the development of the next action potential. Local anesthetics do not interact with closed channels during the resting potential period. Thus, action potentials do not develop in the area where local anesthetics are applied, which is accompanied by a block in the conduction of nerve impulses. The selective effect of local anesthetics on sensory afferent nerves is due to the generation of long-lasting (more than 5 ms) high-frequency action potentials in them.
First of all, local anesthetics block unmyelinated C and myelinated Aδ and Aβ fibers (afferent pathways conducting pain and temperature stimuli; autonomic nerves). Local anesthetics act on fibers surrounded by a myelin sheath at the nodes of Ranvier. Thick myelinated fibers (afferent pathways that conduct tactile stimulation; motor nerves) respond less well to local anesthetics. In addition, the resistance of motor nerves to anesthesia is due to low-frequency short (less than 5 ms) action potentials. In the focus of inflammation, under conditions of acidosis, the dissociation of hydrochloride salts of local anesthetics and the formation of their free lipid-soluble bases are disrupted, so the analgesic effect is lost. For example, local anesthesia may not be effective for tooth extraction in cases of severe periodontitis.
2. General anesthetics
2.1. Inhalational anesthetics
2.1.1 Volatile liquids
Theories of the mechanism of action of general anesthetics
The effects of inhalational anesthetics cannot be explained by a single molecular mechanism. Most likely, the multicomponent action of each anesthetic is realized through many targets. However, these effects converge on a limited number of changes underlying the physiological effects. At the moment, there are lipid and protein theories of anesthesia, but none of them yet describes the sequence of events occurring from the interaction of the anesthetic molecule and its targets to physiological effects.
Figure 2 | Inhalational anesthetics
The activity of inhalational anesthetics is assessed by the minimum alveolar concentration (MAC). A dose producing 1 MAC prevents movement in response to surgery in half of patients. The potency of general anesthetics correlates with their lipid solubility, suggesting the importance of interaction with hydrophilic targets. In particular, the discovery of a relationship between the strength of an anesthetic and its lipophilicity (Meyer-Overton's rule) gave rise to the lipid theory of the mechanism of action of anesthetics. The lipid theory of anesthesia states that anesthetics dissolve in the lipid bilayer of biological membranes and cause anesthesia by reaching a critical concentration in the membrane. The most sophisticated versions of the lipid theory require that anesthetic molecules cause perturbation (changes in properties) of the membrane.
Figure 3 | Meyer-Overton rule
Figure 4 | In the 20th century, it was shown that the potency of general anesthetics correlates with their ability to inhibit the activity of the soluble enzyme luciferase, which is not a physiological target of anesthetics but serves as a lipid-free model protein molecule for anesthetic binding.
Modern facts suggest that proteins, to a greater extent than lipids, are molecular targets for the action of anesthetics. The interaction of anesthetics with hydrophobic regions of proteins also explains the Meyer-Overton rule. The direct interaction of anesthetic molecules with proteins allows us to explain exceptions to this rule, since any sites of binding with a protein are determined both by the size and shape of the molecule, and by solubility. Numerous physical methods (X-ray diffraction, NMR spectroscopy) confirm that general anesthetics act by directly binding to the amphiphilic cavities of protein molecules, and the size of the bound region explains the effect of “chopping off” the properties (longer alcohols lose their anesthetic properties).
Mechanism of action
Thus, general anesthetics change the physicochemical properties of neuronal membrane lipids and disrupt the interaction of lipids with ion channel proteins. At the same time, the transport of sodium ions into neurons is reduced, the output of less hydrated potassium ions is maintained, and the permeability of chloride channels controlled by GABA-A receptors increases by 1.5 times. The result of these effects is hyperpolarization with increased inhibition processes. General anesthetics suppress the entry of calcium ions into neurons by blocking H-cholinergic receptors and NMDA glutamic acid receptors; reduce the mobility of Ca2+ in the membrane, therefore preventing the calcium-dependent release of excitatory neurotransmitters. The most sensitive to the action of general anesthetics are the polysynaptic systems of the central nervous system—the cerebral cortex (1013–1014 synapses), the thalamus, the reticular formation, and the spinal cord. The respiratory and vasomotor centers of the medulla oblongata are resistant to anesthesia.
Volatile inhalational anesthetics:
- postsynaptically enhance inhibitory transmission by potentiating ligand-gated ion channels activated by GABA and glycine;
- extrasynaptically by enhancing GABA receptors and ionic leakage currents;
- presynaptically by increasing basal GABA release.
Inhalational anesthetics inhibit excitatory synaptic transmission by reducing glutamate release and postsynaptically by inhibiting ionotropic glutamate receptors. The paralyzing effect of local anesthetics involves the spinal cord, while sedation/anesthesia and amnesia involve the supraspinal mechanisms of memory, sleep and consciousness.
2.1.2. Gas anesthesia
In anesthesiology, the inhalation gas anesthetic nitrous oxide (N2O) is widely used. At the end of the 1980s. The inert gas xenon entered foreign anesthesiological practice.
Nitrous oxide is a colorless gas with a characteristic odor, stored in metal cylinders under a pressure of 50 atm in a liquid state, does not burn, but supports combustion. Its mixtures with anesthetics of the group of volatile liquids are explosive in certain concentrations. In subnarcotic concentrations (20 - 30%) of nitrous oxide causes euphoria (laughing gas) and strong analgesia. At a concentration of 20% it provides pain relief to the same extent as 15 mg of morphine. Nitrous oxide does not affect GABA-A receptors. Used only in combinations, as the MAC is 104%.
The inert gas xenon is considered the best alternative to nitrous oxide, as it has a more pronounced anesthetic effect, indifference and environmental safety. The ability of xenon to cause anesthesia was discovered in connection with the practice of deep-sea diving and the development of hyperbaric physiology. Xenon is colorless, does not burn and has no odor; upon contact with the mucous membrane of the mouth, it creates a sensation of a bitter metallic taste on the tongue. It is characterized by low viscosity and high solubility in lipids, and is excreted unchanged by the lungs. A xenon-saving anesthesia technology has been developed that includes a minimum flow and a recycling system for repeated reuse of gas. This technology successfully solves the practically important problem of the shortage and high cost of xenon. In the mechanism of the anesthetic effect of xenon, blockade of cytoreceptors of excitatory neurotransmitters - H-cholinergic receptors, NMDA receptors of glutamic acid, as well as activation of receptors of the inhibitory neurotransmitter glycine are important. When interacting with cytoreceptors, xenon acts as a proton-binding cluster and forms complexes with the cations HCO+, NH2+, HNCH+. Xenon exhibits antioxidant and immunostimulant properties, reduces the release of hydrocortisone and adrenaline from the adrenal glands.
2.2. Non-inhalation (intravenous) anesthetics are divided into three groups:
Short-acting drugs (3 - 5 min)
- propanidine (epontol, sombrevin)
- propofol (diprivan, recofol)
Medium-acting drugs (20 - 30 min)
- ketamine (calypsol, ketalar, ketanest)
- midazolam (dormicum, flormidal)
- hexenal (hexobarbital sodium)
- thiopental sodium (pentothal)
Long-acting drugs (0.5 - 2 hours)
- sodium hydroxybutyrate
Figure 5 | General anesthetics for intravenous administration
Mechanism of action
The most widely used intravenous anesthetic is propofol. Its mechanism of action is associated with an increase in chloride conductivity of GABA receptors.
Methohexital is close to propofol in terms of the speed of onset and recovery from anesthesia.
Barbiturates were used for anesthesia before the introduction of propofol. Thiopental causes rapid onset and rapid recovery from anesthesia with a single injection, but it accumulates rapidly with repeated or prolonged administration and thus slows recovery from anesthesia. Barbiturates are ligands of barbiturate receptors. In small doses, they allosterically enhance the effect of GABA on GABA A receptors. At the same time, the open state of chlorine channels lengthens, the entry of chlorine anions into neurons increases, and hyperpolarization and inhibition develop. In large doses, barbiturates directly increase the chloride permeability of neuronal membranes. In addition, they inhibit the release of excitatory mediators of the central nervous system - acetylcholine and glutamic acid, and block AMPA receptors ( quisqualate receptors) of glutamic acid. Barbiturates have cerebroprotective properties and can be used for this purpose.
Benzodiazepines are used mainly as anxiolytics and for sedation with preservation of consciousness. All benzodiazepine receptors allosterically enhance the cooperation of GABA with GABA A receptors, which is accompanied by an increase in the chloride conductivity of neurons, the development of hyperpolarization and inhibition. The reaction with benzodiazepine receptors occurs only in the presence of GABA. Remimazolam is the youngest benzodiazepine and has a very short duration of action due to rapid neutralization by plasma esterases.
Ketamine is chemically a derivative of phencyclidine. The synaptic mechanisms of action of ketamine are diverse. It is a non-competitive antagonist of the excitatory brain mediators glutamic and aspartic acids in relation to NMDA receptors. These receptors activate sodium, potassium and calcium channels in neuronal membranes. When receptors are blocked, depolarization is disrupted. In addition, ketamine stimulates the release of enkephalins and β-endorphin; inhibits the neuronal uptake of serotonin and norepinephrine. The latter effect is manifested by tachycardia, an increase in blood pressure and intracranial pressure. Ketamine dilates the bronchi. When recovering from ketamine anesthesia, delirium, hallucinations, and motor agitation are possible (these adverse events are prevented by the administration of droperidol or tranquilizers). An important therapeutic effect of ketamine is neuroprotective. As is known, in the first minutes of brain hypoxia, excitatory mediators - glutamic and aspartic acids - are released. Subsequent activation of NMDA receptors, increasing the concentration of sodium and calcium ions and osmotic pressure in the intracellular environment, causes swelling and death of neurons. Ketamine, as an antagonist of NMDA receptors, eliminates ion overload of neurons and the associated neurological deficit.
The newest intravenous anesthetic is dexmedetomidine. It is a highly selective α2-adrenergic receptor agonist with sedative, sympatholytic, hypnotic and analgesic effects. Its main action is as an agonist at the a2 receptors in the locus coeruleus.
Sources:
- Theories of the mechanism of action - https://en.wikipedia.org/wiki/Theories_of_general_anaesthetic_action and https://www.esus.ru/php/content.php?id=744
- Vengerovsky A.I., Lectures on pharmacology for doctors and pharmacists 2007
- Kharkevich, Pharmacology ed. 10
- Miller's anesthesia / Ronald D. Miller; associate editors, Neal H. Cohen, Lars I. Eriksson, Lee A. Fleisher, Jeanine P. Wiener-Kronish, William L. Young. - Eighth edition. 2015
It is impossible to imagine modern dentistry without local anesthesia. Most therapeutic, some orthopedic and almost all surgical interventions are performed after preliminary anesthesia. Articaine-containing local anesthetics, widely used and deservedly considered the most effective and safe, allow many complications to be avoided if the rules of their use are followed correctly [3]. But they also use lidocaine and even procaine (Novocaine), and in maxillofacial surgery - ropivacaine for long-term intra- and postoperative pain relief, so doctors should be well aware of how great the risk of complications is directly associated with the use of local anesthetics (see diagram) .
Let us dwell on systemic toxic reactions to local anesthetics - one of the little-studied complications that can lead to a critical outcome. In contrast to allergic reactions, systemic toxic reactions to local anesthetics are common, especially when high doses of drugs are used. Over the past decades, scientists around the world have carried out a great deal of research and analytical work to study the toxic effect of local anesthetics on the human body and determine the leading signs that distinguish this complication from allergic reactions. The published results of statistical studies convincingly indicate that modern local anesthetic drugs are much less dangerous as allergens (the risk of allergy is 1:2,000,000 injections) and much more likely to cause systemic toxic reactions (the risk is estimated as 1:1000 injections) [12].
Systemic toxic reactions to local anesthetics in maxillofacial surgery and dental practice, in contrast to general surgery, where these drugs are administered in much larger quantities for the purpose of regional anesthesia, are less common, but this complication must be remembered due to the likelihood of a tragic outcome. .
The modern market provides dentists with a wide selection of local anesthetics. When determining the safest drug, it is important to focus primarily on its anesthetic index, duration of effective action and compatibility with other drugs taken by the patient. In table 1 provides information on the maximum doses, duration of action, activity and toxicity of local anesthetics.
Table 1. Anesthetic activity, duration of action, toxicity and maximum permissible dose of various local anesthetics Note. m/a - local anesthetic; m/a+iv/c - local anesthetic with vasoconstrictor; activity - the ratio of the minimum effective dose of novocaine to the minimum effective dose of another drug; toxicity - the ratio of the minimum lethal dose of novocaine to the minimum lethal dose of another drug; anesthetic index is the ratio of the potency of the drug to the relative toxicity of the drug.
The analgesic effect of a local anesthetic is determined by its vasoactivity, lipid solubility, ability to bind to proteins and acidity. Certain adjustments, of course, are made by the somatic status of the patients, as well as the pharmacological interactions that occur in the case of basic drug therapy.
The rate of onset of the analgesic effect, systemic absorption, metabolism and elimination of the local anesthetic determine its physicochemical properties. Thus, the lipid solubility and protein-binding characteristics of a local anesthetic depend on its affinity for the cell membrane of the neuron, which consists of fat (90%) and protein (10%). The higher the fat solubility of the drug, the easier it penetrates the membrane, and therefore the more effective. The better the anesthetic binds to protein, the longer its duration of action. The dissociation constant of the drug determines the rate of onset of the analgesic effect, as well as absorption and elimination.
After injection, part of the anesthetic diffuses onto the neuron membrane; the remaining drug enters the vascular bed. Increased absorption of local anesthetic equates to direct entry into the vessel; This should be especially feared in the acidic environment of the inflammatory focus. The amount of drug absorbed by the vessels depends on vascularization and the amount of blood flow in the anesthetized tissue, as well as the effect of the local anesthetic on the tone of the vascular wall. During infiltration anesthesia, amide anesthetics of medium and long action cause vasodilation, and ropivacaine (naropin), unlike other long-acting drugs (bupivacaine hydrochloride - marcaine), gives a vasoconstrictor effect. If a local anesthetic drug contains a vasoconstrictor, the rate of systemic absorption slows down, but there is a risk of developing tachycardia and atrial fibrillation.
Until recently, it was believed that the degree of systemic toxicity was strictly dependent on the concentration and amount of anesthetic administered. Thus, using lidocaine as an example (Fig. 1), peak levels of anesthetic concentrations in blood plasma were shown, which determine the possible clinical picture of a systemic toxic reaction.
Rice. 1. Dependence of the clinical manifestations of the systemic toxic reaction of lidocaine on the level of its plasma concentration.
However, according to recent publications, the described cases of systemic toxicity of local anesthetics in the practice of general and maxillofacial surgery have suggested that the manifestation of toxic reactions does not always depend on the excessive amount of administered anesthetic; even the doses of drugs recommended for use can cause this complication [6, 16, 18].
Clinically, the systemic toxic effect of local anesthetics is more often manifested by symptoms of central nervous system damage and less often by cardiovascular damage. Due to the variety of conditions that determine the timing and rate of entry of the local anesthetic drug into the vascular bed, the period of development of clinical symptoms of systemic toxicity varies within a fairly wide range - from 1-5 to >60 minutes [10].
Based on the severity of clinical symptoms, systemic toxicity of local anesthetics is divided into 3 degrees [2]. If its degree is mild (minor toxic reactions), the patient’s first complaints are tingling and itching in the area where the anesthetic was administered; these sensations may spread to the lips and tongue. At the same time, tinnitus and a metallic taste in the mouth appear. Breathing and hemodynamics are not impaired, moderate tachycardia may be observed. Since the patient's consciousness is preserved, changes in well-being, as a rule, do not go unnoticed; they cause the patient a certain anxiety, which increases as the symptoms intensify; a feeling of fear is often added. The clinical picture of the development of a toxic reaction may be limited to this; gradually the unpleasant sensations disappear without additional therapeutic measures, and the patient calms down. Most often, mild complications of local anesthesia go unnoticed by the doctor and are not recorded in the medical history. But if the patient’s subjective sensations are accompanied by trembling, twitching of individual muscles, nausea, possibly vomiting, and signs of impaired consciousness, in particular orientation, are noted, it should be understood that the toxic reaction develops and becomes moderately severe. With the maximum severity of clinical manifestations at this stage, the patient’s consciousness becomes confused, speech is impaired, numbness and retardation are characteristic, rapid breathing, decreased blood pressure, bradycardia, motor agitation or convulsions are noted. The appearance of seizures indicates that the systemic toxic reaction is becoming severe. Against the background of convulsions, loss of consciousness, decreased muscle tone, respiratory failure up to apnea, arrhythmias, asystole, relaxation and paralysis of the sphincters can occur. Without providing emergency care to patients in critical condition, including resuscitation measures with mandatory specific elements, death is inevitable.
A systemic toxic reaction to local anesthetics is not necessarily clinically manifested by all of the listed signs; some of them are often observed. An analysis of 93 cases of systemic toxicity of local anesthetics showed that the classic clinical picture developed in only 60% of victims; the predominance of pathological signs of the central nervous system with cardiovascular disorders is observed in 30.3% of cases, and only isolated cardiovascular disorders are described in 9.7% of patients [10].
The frequency of clinical signs indicating the development of a systemic toxic reaction following accidental intravascular administration of a local anesthetic is presented in Fig. 2.
Rice. 2. Frequency of development of clinical signs of toxic effects of local anesthetics [7].
Due to the complexity of conducting studies (it is impossible to perform them on humans!), the mechanism of development of systemic toxicity remains unclear. Today it is known that local anesthetics inhibit the process of oxidative phosphorylation, so the most severe disorders occur in the organs that are least adapted to anaerobic respiration metabolism, and this is primarily the brain and heart [2].
It is believed that the toxic effect on brain cells determines the ability of a local anesthetic to penetrate the blood-brain barrier and block sodium channels of excitable cell membranes, which causes toxic reactions of varying severity. If a convulsive syndrome occurs, the accompanying metabolic acidosis increases the uptake of the drug not only by brain neurons, but also by cardiomyocytes.
Cardiotoxicity is probably primarily determined by the ability of local anesthetics to reversibly bind to the intracellular part of the voltage-gated sodium channels of the cardiomyocyte, preventing them from opening and thereby preventing the generation of an action potential and its propagation along the nerve fiber. And the higher this ability of the anesthetic, the stronger its analgesic effect, but at the same time, the inhibitory effect on the conduction system of the heart [8].
All local anesthetics in high concentrations increase the refractory period of the heart, inhibit the excitability, contractility and conductivity of the myocardium - which means that in doses exceeding permissible concentrations, any of the local anesthetic drugs can cause severe depression of the myocardium. It is important to note that the clinical picture of systemic toxicity caused by lidocaine is manifested primarily by severe pathological reactions from the central nervous system; this allows the doctor to take the necessary measures to save the patient in time. And drugs that are more powerful than lidocaine, even in what is considered an acceptable plasma concentration, can, without causing convulsive manifestations or reducing myocardial contractility, provoke arrhythmias of varying severity [8].
Particular care when performing local anesthesia is required in patients with risk factors for systemic toxicity; these include anemia; hypoproteinemia; chronic heart failure with congestion; impaired liver and kidney function; endocrine and metabolic diseases; pregnancy; old age and young age (children). Compared with adults, young children have a significantly higher ratio of cardiac output and regional blood flow to body weight, and systemic toxic reactions develop more quickly in children due to the rapid increase in peak toxic drug concentrations in plasma. Thus, in a 3-year-old child after topical anesthesia, almost instantaneous absorption of the local anesthetic occurs, and the time for the onset of peak plasma concentration of lidocaine during infiltration anesthesia is comparable to that during its intravenous administration.
Special attention should be paid to the treatment of patients under sedation and general anesthesia using local anesthetics. The literature [10] describes 2 cases of systemic toxicity in infants 6 and 9 months old during uranoplasty under general anesthesia a few minutes after infiltration of local anesthetic (in one case, lidocaine, in the other, articaine). The emerging convulsive syndrome left no doubt about the diagnosis, and assistance was provided in a timely manner. Upon completion of postoperative rehabilitation, patients were discharged from the hospital without neurological deficit. Analysis of other episodes of systemic toxicity occurring during general anesthesia shows that signs of cardiovascular complications are more common because the central nervous system is blocked and early symptoms go unnoticed.
Prevention of toxic effects of local anesthetics
A systemic toxic reaction can be anticipated in cases of planned treatment of patients at risk, when the recommended dose of anesthetics is exceeded, or in non-compliance with anesthesia techniques. But, unfortunately, there is no guaranteed option to prevent this complication [15].
Prevention of systemic toxicity consists of strict adherence to instructions for the use of local anesthetics, correct execution of anesthesia techniques and careful attention to the patient both at the stage of preparation for treatment and during it.
During the initial consultation, be sure to find out complete information about the patient’s health. If you have concomitant somatic diseases, obtain an extract from the medical history indicating the diagnosis and the basic therapy recommended for use. If necessary, conduct additional examination. Before treatment, be sure to determine the patient’s physical condition and record the main parameters (blood pressure, heart rate, respiratory rate) in the medical history. Be sure to inform the patient about the possible risks and obtain his written consent for all planned interventions [3].
When choosing a local anesthetic drug, be sure to choose the least toxic of them, if the situation allows it. The use of long-acting local anesthetics is justified for long-term interventions that require prolonged postoperative pain relief, and only if a full set of resuscitation equipment is available.
Be sure to use the minimum effective dose of local anesthetic, choose a sufficient concentration and do not exceed the required volume of anesthetic. Consider the absorption capacity of the surrounding tissues at the injection site [4].
Before administering the anesthetic, be sure to perform an aspiration test and repeat it if you move the needle along the injection; Do not forget that the results of the aspiration test are false negative in approximately 2% of cases. To increase safety, use ultrasound guidance during injection whenever possible, although its effectiveness has not yet been studied and the risk of intravascular injection of anesthetic remains [5].
Be sure to inject the local anesthetic gradually, slowly (1 ml/min) or fractionally with pauses of 15-30 s. When introducing large portions of anesthetic, the injection interval must be increased to reduce accumulation [4].
Do not interrupt verbal contact with the patient during local anesthesia; be sure to monitor his reaction during manipulation and actively identify complaints - this increases the likelihood that the first subjective signs of a developing systemic toxic reaction will be noticed by you on time [3].
Below are the maximum permissible doses of local anesthetics most used in outpatient dental practice for children and adults (Tables 2-5).
Table 2. Maximum permissible doses of lidocaine 2%
Table 3. Maximum permissible doses of mepivacaine 2%
Table 4. Maximum permissible doses of articaine 4%
Table 5. Maximum permissible doses of mepivacaine 3%
Therapeutic measures for systemic toxic reactions
Until recently, the treatment of systemic toxic disorders caused by local anesthetics included the use of anticonvulsants, ensuring adequate oxygenation of the patient up to tracheal intubation followed by artificial ventilation, infusion therapy with large volumes of blood substitutes with the introduction of glucocorticosteroids and drugs that correct cardiovascular disorders. Carrying out cardiopulmonary-cerebral resuscitation, if necessary, did not include any special features; the complex was performed according to the general protocol [1].
Currently, along with measures to replace the patient’s vital functions, the priority in the treatment of systemic toxicity is to reduce and, if possible, eliminate the systemic effect of local anesthetics [13]. The recommended intravenous administration of lipid solutions, the effectiveness of which has been proven experimentally and clinically [9], allows the symptoms of toxicity to be relieved by binding lipophilic local anesthetics. The plasma concentration of drugs decreases, they are “washed out” (detached along the concentration gradient from the membrane of the neuron and cardiomyocyte) and removed with new portions of lipid. In addition, it is known that lipid-containing solutions are an energy substrate for cardiac mitochondria and this ability is in demand in the case of cardiotoxic reactions. The treatment regimen using Intralipid solution (20%) to eliminate systemic toxic reactions of local anesthetics was called “lipid rescue” [7].
The clinical picture of the severe stage of systemic toxicity of local anesthetics can develop rapidly, so it is very important to correctly assess the first symptoms and begin emergency care in a timely manner. Seizure syndrome remains an absolute indication for the use of benzodiazepines; it is more dangerous to treat it with propofol or ultra-short-acting barbiturates due to their own pronounced cardiodepressive effect. As in previous recommendations, one of the first measures to save the patient is to ensure adequate oxygenation by any available means, including tracheal intubation and mechanical ventilation with 100% oxygen. In case of ineffective blood circulation due to arrhythmia or asystole, resuscitation measures should be started immediately and continued for at least 60 minutes. Since local anesthetics do not cause irreversible changes in the myocardium, with sufficient oxygenation of the brain and the administration of lipid solutions during this time, there is hope for saving the patient. It is extremely important not to rush to stop effective resuscitation measures if systemic toxicity is caused by powerful anesthetics, because they take a long time to “wash out” [6]. When carrying out symptomatic therapy, if necessary, it is necessary to avoid the administration of lipophilic drugs - beta-blockers and calcium channel blockers, abandon vasopressin, use only amiodarone (cordarone) to relieve ventricular arrhythmias, reduce the dosage of epinephrine (adrenaline) to less than 0.1 mcg/day. kg or completely abandon its administration [13].
Literature sources [20] report that in case of a systemic toxic reaction to local anesthetics, rapid administration of large volumes of lipid solutions is not accompanied by significant complications, and their clinical effectiveness against the background of resuscitation measures allows one to avoid residual cardiac disorders and neurological deficits [10, 17].
For “lipid leaching,” any fat emulsion can be used, but Intralipid (20%) has proven itself to be the best [11]. Inactivation of local anesthetics with diprivan (propofol) should not be carried out due to the need for a large amount of the drug to obtain a positive result due to its low lipid content, and this is dangerous since diprivan has a direct cardiodepressive effect [15]. Today, it is recommended to relieve a systemic toxic reaction to local anesthetics with lipids, starting the administration of the drug when the first characteristic complaints or symptoms appear and at the same time being ready to perform a full range of resuscitation measures [6].
In each medical institution, including dental, where local anesthetics are used, it is necessary to have 1 liter of fat emulsion solution (Intralipid 20% - 500 ml or other), syringes for “lipid washing out” with a volume of 50 ml, peripheral intravenous catheters 14-16 G, Infusion systems, copy of the Lipid Rescue protocol [19].
After catheterization of the peripheral vein, the lipid solution must be administered as a bolus (Intralipid 20% - 1.5 ml/kg) using 50 ml syringes, then switch to drip infusion (Intralipid 20%, rate - 0.25 ml/kg/min) and Continue administering the drug at this pace for at least 10 minutes after blood circulation has stabilized. If it is not possible to stabilize blood circulation with this dose of lipid solution, it is necessary to repeat its bolus administration in the same dosage (Intralipid 20% - 1.5 ml/kg) and switch to an accelerated infusion of the drug (Intralipid 20%, rate - 0.5 ml/kg /min). The highest single dose of fat emulsion is acceptable - 10 ml/kg/min.
At the Euroanaesthesia 2010 forum in Helsinki (Finland), the European Board of Anesthesiology, together with the European Society of Anaesthesiology, adopted a declaration on patient safety in anesthesiology. It provides fundamental points that make it possible to prevent and avoid serious complications and prevent danger to patients. In particular, it is stated that all medical institutions that use local anesthetics “... must have protocols and facilities necessary in situations of systemic toxicity...” [14]. The Declaration of Helsinki has been endorsed by the World Health Organization, the World Federation of Societies of Anaesthesiologists and the European Patients Federation. Taking into account its specifics, each country has the right to refine and adapt the protocol for resuscitation using lipid solutions to its own conditions. We consider this task extremely important for our medical community.
The authors declare no conflict of interest.
Preparing for local anesthesia
If the patient is undergoing surgery with local anesthesia, the doctor should explain in advance how to prepare.
Patients should tell their doctor if they are using any medications, especially if they are blood thinning agents such as aspirin or warfarin.
Your doctor may instruct you not to eat anything for several hours before surgery. It is also important not to drink alcohol for 24 hours before taking the anesthetic.
In the doctor's office, the doctor applies a local anesthetic to the appropriate area of the body. It will start to feel numb.
The doctor will not act if the patient does not feel numb.
The anesthetic will prevent any pain, but the patient may still feel pressure during surgery.
Depending on what the procedure is and how anxious the patient feels, a sedative may be prescribed at the same time. This will help the patient feel calm and less anxious.
The doctor will monitor the amount of oxygen in your blood using a small device placed on your finger. In rare cases, a plastic nasal tube will be used to provide supplemental oxygen.
Risks and complications
Local anesthesia is generally considered very safe. For minor surgeries it is safer than general anesthesia.
There may be tingling and pain, and there may be bruising as you take the drug and wear it off, but this is usually minor.
A person who has had local anesthesia must be careful not to get hurt when they cannot feel pain, such as when biting their cheek after dental treatment.
Temporary side effects that affect some people include:
- blurred vision, dizziness and vomiting
- headache
- muscle twitching
- persistent numbness, weakness, or tingling
Some people may have an allergic reaction. The patient may experience hives, itching, and difficulty breathing.
Cyanosis may occur, in which the skin turns bluish due to poor circulation or insufficient oxygenation of the blood.
In very severe cases, a person may experience CNS depression, in which the central nervous system slows down too much, resulting in a decrease in breathing and heart rate. This can lead to cardiac arrest if blood stops pumping to the heart.
An overdose of local anesthetic can lead to seizures. This can be life-threatening.