Many dental clinics offer fillings using several different filling materials. Price lists (including those posted on websites) are also often replete with various commercial names of fillings: Filtek, Spectrum, Aesthetics, Charisma (Karizma), Vines (Venus), Gradia, Estelite, Herculite, Point, Tetrik, Admira, Brilliant, Enamel Plus and others. Patients are asked to make a choice, but this requires dental-level knowledge, which most people, of course, do not possess.
Popular articles on the Internet for the following queries: “which filling is better?”, “which filling material is the best?”, “which filling is better to put?” and so on. – do little to clarify the situation. Most articles (often written by anonymous authors or copywriters) discuss only classes of filling materials: cements, amalgams, glass ionomers, chemical and light-curing composites. After a detailed description of materials that went out of use several decades ago (apparently to give more volume to the text), the conclusion is made that light-curing composites are the best, and if a person has the financial means, then it is advisable to install fillings from them. Either nothing is mentioned about specific brands of heliocomposites, or it is stated that they are all approximately equally good. Or “the latest generation nanocomposites” begin to be advertised.
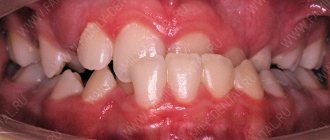
What fillings do not need to be placed?
The first and most important thing that every patient should understand is that light-curing fillings (photopolymers, heliocomposites) are the only reasonable choice in the 21st century. Even for people on a budget. Other materials are not 10 or 20 percent, but many times worse. Discussing the advantages or price/quality ratio of cement fillings in our time is as stupid as looking for advantages, for example, in reel-to-reel tape recorders. For a long time now, no one has been producing or buying cassette and video recorders, but for some reason some individuals allow themselves to install a 19th-century seal.
Plastic fillings are completely beyond reason. Acrylic oxide and similar anachronisms should have been banned for use a quarter of a century ago, since they are not only unable to stop the further spread of caries, but also provoke its progression. A plastic filling on a tooth is a direct road to pulpitis and periodontitis. You have to hate yourself with a fierce hatred to allow this to happen to you. And clinics that currently use such materials will be deprived of their license.
Amalgam fillings are famous for their durability, but due to toxicity (not for the patient, but for the environment and medical staff), dentists themselves refuse them.
Chemical composites are moderately bad - if a person lives from hand to mouth, then, of course, you can install them (it’s still better than nothing). But for most other people who do not experience urgent need every day, this will not be a justifiable choice. It may happen that such a filling will last for many years, but more often the opposite happens. After 3-5 years (or earlier), caries will develop under the “chemical” filling, and it can either reach pulpitis, or the amount of destruction will become so great that instead of re-installing the filling, the manufacture of an inlay or onlay will be required. As a result, the cost of repeated intervention increases by an order of magnitude due to the savings of thousands of rubles the first time (the approximate difference with the cost of a light-curing composite).
Indications and contraindications
Due to its versatility, this composite can be used in quite a few procedures for the prevention and treatment of dental diseases. Estelite can be used:
- for carrying out the restoration procedure of the frontal zone of the jaw row;
- to eliminate minor external defects from the tooth surface;
- as a base layer;
- in treatment processes using the indirect restoration method;
- to restore artificial tooth fragments;
- as a material for eliminating cracks and diastemas;
- as a component of composite veneers;
- for the prevention and treatment of caries.
The presence of methacrylic monomers in Estelite suggests contraindications for those patients who have an individual intolerance to these substances.
Therefore, before use, you must make sure that this drug will not cause an allergic reaction.
Preparation for use
The process of preparing the oral cavity for appropriate procedures is a necessary and important part of a positive result. Tooth preparation is carried out only in the specified order and includes the following medical procedures:
- Enamel cleaning
– the entire surface of the teeth must be cleaned using a special fluoride-free mass that fits in a rubber container, and then rinsed with water.
- Selecting a color shade
– there is a special shade scale, but to choose the optimal color it is recommended to wait a little, because dry teeth have the same color, while wet teeth are slightly different.
To correctly choose the optimal color option, you should choose the most intense one from those selected.
Attention!
Under no circumstances should professional teeth whitening be performed before these procedures. Otherwise, it may happen that the shade will be quite different after some time. It is also not recommended to use eugenol and its derivatives for protective functions, since then the polymerization process of the presented composite is much worse.
Restoration process step by step
It is recommended to use unidoses in the manner described in the instructions. Before use, an ounce of material is placed in the dispenser, then the cap is removed, which does not allow the material to flow out. The composite is filled directly into the indicated cavity or prepared for application on a special tablet, bringing it to the desired state.
When using a syringe, the following manipulations are performed:
- you need to remove the protective cap;
- turn the handle clockwise;
- place a small amount of mass on a special tablet;
- knead the paste.
Once the material reaches the desired level of consistency, filling and restoration work can be carried out. The paste should be applied in small doses, in layers, so as not to exceed the permissible depth. To guide the dosage, the creators of Estelite have developed a special table.
When the filling process is carried out using the Estelite composite mass, it is prohibited to use other preparations, since the possibility of voids increases and the polymerization process will not proceed properly.
Each layer is polymerized separately, the time specified by the manufacturer. And the surface treatment completes the procedure. The site for restoration work is cleaned and a small cosmetic polishing is carried out using burs with a fine-grained diamond cut.
In order to erase the boundaries between the tooth surface and the filling, the dentist performs a polishing procedure with a multifaceted carbide bur. This does not require water and rotation is minimized.
Palette of material shades
Estelite filling material is available in a very wide range of shades - A1, A2, A3, A3.5, A4, A5, B1, B2, B3, B4, C1, C2, C3, OA1, OA2, OA3, OPA2, BW (created for for filling whitened teeth), CE (for enamel types with increased transparency, cutting edge shade) and WE (white enamel).
The opalescent shades in the product line, which include OA1, OA2 and OA3, are distinguished by a fairly good level of opacity. Thanks to this, Estelite is 100% suitable for blocking the darkness of the oral cavity (this makes it possible to carry out restoration work on cavities of classes 3 and 4).
Opalescent shades are also actively used as dentin shades when restoration procedures are carried out with several different shades in several layers. Shades 4FA1, OA2 and BW are used during restoration work on primary teeth. However, they are practically not used in cases where it is necessary to perform a restoration in which pins and metal structures were used. As well as those teeth whose color has changed significantly. For example, tetracycline teeth.
OPA2 is a color used to hide small enamel stains. The Estelite material can also be used to restore the patient’s opaque teeth.
Light-curing fillings are not uniform
Glass ionomer cements with a light-initiated curing mechanism are available. For example, Fuji 2 (Fuji II LC). Compomers (a hybrid material between composites and glass ionomers) are also cured using a lamp. Their most famous representative is Dyract XP. These materials are more reliable than some heliocomposites, but are still significantly inferior to their best representatives. Due to the misconception about the toxicity of composites, some doctors recommend these materials for filling for children. For pediatric dentistry, Fuji and Direct are acceptable options, but composites are still preferable.
Dental composite materials are divided into universal (suitable for filling any cavities), aesthetic for anterior teeth (excellently convey all the nuances of the color of natural teeth) and packable for chewing teeth (have low shrinkage and abrasion, but also low aesthetic characteristics). There is another class of composites – flowable. Previously, they were recommended for filling wedge-shaped defects due to their high elasticity, however, according to modern data, universal composites give better results even with non-carious lesions.1 Therefore, current composites currently have only an auxiliary value: they are used together with other classes of materials, but the main one the volume of the filling does not make up.
Universal heliocomposites are the most widely used type of filling materials in the world. The commercial names of fillings offered to the patient to choose from mostly refer to these materials. They also differ in quality. The percentage of inorganic filler, its chemical composition and the shape of the granules determine the mechanical properties of the filling: polymerization shrinkage, mechanical strength, hardness and wear resistance, polishability and surface smoothness. Some of these parameters antagonize each other. For example, for good polishability, the granules of non-limiting filler (oxides of silicon, zirconium, aluminum, etc.) should be as small as possible, and to increase strength, on the contrary, as large as possible. Therefore, materials advertised as “nanocomposites” cause a wry smile among professionals - the prefix “nano-” attracts illiterate dentists and patients who blindly associate it with high technology. However, for the sake of fairness, quite good material can be presented. For example, Filtek Ultimate.
Glass ionomer cements (glass ionomers)
Glass ionomer cements (GIC) are a whole class of modern dental materials created by combining the properties of silicate and polyacrylic systems. Filling teeth using glass ionomer cements is gradually replacing zinc phosphate and zinc polycarboxylate cements from dental practice. Glass ionomer cements are usually classified according to a number of characteristics.
According to their application. For permanent fillings (aesthetic, reinforced), quick-hardening (for gaskets, fissure sealing), for filling root canals, for fixing orthopedic structures.
- By release form:
- powder-liquid (powder – finely dispersed aluminofluorosilicate glass with various additives, liquid – an aqueous solution of a copolymer of carboxylic acids with the addition of tartaric acid);
- powder (all components are in powder, which is mixed with distilled water; so-called Aqua cements);
- capsules (powder and liquid are packaged in capsules with a thin partition in the required ratio, so when mixed, glass ionomer cement with optimal properties is obtained);
- paste (in tubes or syringes); do not require mixing and harden when irradiated with a halogen lamp.
Depending on the chemical composition of the curing mechanism.
- Classic (powder-liquid). Finely dispersed aluminum fluorosilicate glass powder (particle sizes 20-50 microns). Powder components: silicon dioxide, aluminum oxide, calcium fluoride, fluorides of other metals (providing fluoride release for caries prevention), aluminum phosphate (providing strength and abrasion resistance), barium, zinc, strontium salts, etc. (providing radiopacity). The liquid is an aqueous solution of a copolymer of polycarboxylic acids (acrylic, itaconic, maleic) with the addition of a tartaric acid isomer. In the case of Aqua cements (only powder that is mixed with distilled water), polycarboxylic acids are included in the original powder in the form of crystals. In metal-containing glass ionomer cements, metal additives and alloys (silver-tin, silver-palladium) are additionally introduced into the powder composition. Hardening of classic glass ionomer cements occurs according to the type of ion exchange reaction (hence the name glass ionomer): hydrogen ions (present in an aqueous solution of polycarboxylic acids) exchange with metal ions (calcium, aluminum) of the glass, calcium and aluminum ions bind the hydroxyl groups of polycarboxylic acid chains (a matrix is formed metal polyacrylate containing unreacted glass particles). In the initial stage of hardening, calcium polyacrylic chains are formed quite quickly. This reaction ensures the cement sets and lasts for several minutes. However, the efficiency of binding by calcium ions is not high enough and in the early stages of hardening, calcium-polyacrylic chains can dissolve in water (therefore, the cement must be protected from moisture during this time). When the calcium ions have reacted, aluminum ions react and aluminum-polyacrylic chains are formed. The trivalent nature of aluminum (as opposed to the divalent nature of calcium) provides a higher degree of cross-linking and the formation of spatial structure. It is at this stage that the final cement matrix is formed. The completion of the second phase occurs in approximately 2-3 weeks (the curing process can be accelerated by the use of hybrid glass ionomers, which already gain sufficient strength within about 40 seconds at the initial stage of photopolymerization). Additionally, silica gel (strong structure) is formed on the surface of the glass particles. As a result, the final structure of hardened glass ionomer cement is glass particles surrounded by silica gel and located in a matrix of cross-linked polycarboxylic acid (metal polyacrylate) molecules.
- Hybrid glass ionomer cements (polymer modified glass ionomer cements). They have a double (chemical and light) or triple hardening mechanism. The powder is finely dispersed aluminosilicate glass (as in the case of classical glass ionomer cements), sometimes with the addition of polycarboxylic acid copolymer crystals (as in the case of Aqua cements). The liquid is an aqueous solution of a copolymer of polycarboxylic acids (acrylic, itaconic, maleic), the ends of the molecules of which are modified by the addition of unsaturated methacrylate groups (as in dimethacrylates of composite filling materials). The liquid also contains tartaric acid, hydroxyethyl methacrylate and camphoroquinone (a photoinitiator). The first stage of the curing mechanism is the binding reaction of the terminal unsaturated methacrylate groups of polycarboxylic acids due to the photoinitiated formation of terminal radicals (photopolymerization). The second stage is the usual classical reaction of cross-linking of polyacid macromolecules with metal ions. Hybrid glass ionomer cements (with a dual curing mechanism) have improved physicochemical properties, but also have a significant drawback: in areas inaccessible to light from a photopolymerizing lamp, curing occurs only due to a classical chemical reaction (which affects the physicochemical characteristics of glass ionomer cements) . Glass ionomer cements with a triple curing mechanism do not have this drawback (the first two stages are like double-curing glass ionomer cements, and the third stage is catalytically initiated polymerization of the terminal methacrylate groups of polycarboxylic acids without exposure to light).
This classification is conditional, since many modified glass ionomer cements have recently appeared: with the addition of polymer resins, with specially treated fine glass particles, etc.
A very important advantage of glass ionomer cements is good chemical adhesion to tooth tissues. This is believed to occur due to the formation of chelate bonds between the hydroxyl groups of polycarboxylic acids and calcium ions of the surface hydroxyapatite (similar to the classical chemical cross-linking reaction during the hardening of glass ionomer cements), as well as due to the formation of hydrogen bonds of carboxylate groups with collagen (an organic component of dental tissues).
Other advantages of glass ionomer cements include good chemical adhesion to other filling materials (including composites), high biological compatibility with tooth tissues, thermal expansion characteristics close to tooth tissues (which protects against violation of the marginal seal of fillings), low modulus of elasticity ( which allows the use of glass ionomer cements as spacers or a base for dental restoration with composite materials).
Glass ionomer cements have bioactivity, which is associated not only with chemical adhesion to tooth structures, but also with prolonged fluoride release and the release of other ions (aluminum, calcium, strontium; they contribute to the remineralization of tooth structures during carious lesions). All other restorative materials (for example, composites) are not bioactive and serve only to restore the shape and aesthetics of the tooth. During the initial period (about 2 days) of hardening of glass ionomer cements, fluoride ions are rapidly released, which remain free within the glass ionomer matrix. The free movement (diffusion) of fluoride ions is due to the fact that they are not structurally associated with the cement matrix and are capable of migration into the oral cavity and into the dental tissue adjacent to the restoration (filling), while providing a cariesostatic and antibacterial effect. The release of fluoride ions (in smaller quantities) continues over a long period (prolonged process, more than 1 year). The diffusion of fluoride ions into dentin and enamel causes increased mineralization of hard tooth tissues, a decrease in dentin permeability, remineralization of initial carious lesions and stopping or slowing down the remaining carious process. The hard tissue under glass ionomer cement turns out to be denser and hypermineralized. In addition, glass ionomer cements are capable of adsorbing (absorbing) fluoride ions upon contact with fluoride-containing materials (toothpastes, gels, rinses), which leads to re-enrichment of the glass ionomer restoration (filling) with fluoride ions. The incoming fluoride ions are then slowly released into the oral cavity and tooth tissue adjacent to the restoration (filling). Thus, glass ionomer cement acts as a reservoir (depot) of fluoride ions. In recent years, glass ionomer cements are increasingly used to seal fissures (primarily due to the remineralizing effect on the enamel in the fissure area due to fluoride release).
Typical representatives of modern glass ionomer cements are the following.
Fuji Plus is a composite-reinforced glass ionomer cement. Used for permanent cementation of metal, metal-ceramic and metal-composite crowns and bridges, inlays and onlays made of composites, ceramics and dental alloys.
Fuji I (Fuji I) is a glass ionomer cement for permanent cementation of orthopedic crowns, bridges, inlays, onlays made of any dental alloys.
Fuji IX (Fuji 9, Fuji IX) is a classic glass ionomer restoration (filling) cement of packable viscosity (the term “packable” means maintaining the shape given to the material even before the stage of its curing, which allows the dentist to easily carry out the preliminary modeling stage). Due to its high abrasion resistance, it is used for restorations (fillings) in the area of chewing teeth and reconstruction of the crown part of the tooth.
Fuji Lining is a light-curing glass ionomer cement. It has low shrinkage during hardening, therefore it is used as an insulating gasket.
Ionosit Baseliner is a light-curing hybrid glass ionomer cement (more often referred to as compomers). A one-component material that expands slightly when curing and is therefore used as an insulating spacer to compensate for polymerization shrinkage of composites. Physical properties are approximately 3 times stronger than traditional glass ionomer cements.
TimeLine is a light-curing glass ionomer material. Used as an insulating spacer for composite fillings (restorations).
CORE MAX is a glass ionomer cement reinforced with a composite (sometimes referred to as chemically cured composites). Particularly strong cement for restoring the crown of a tooth using pins. RelyX LUTING is a chemically cured hybrid glass ionomer cement. Used for permanent cementation of orthopedic crowns, inlays made of ceramics, metals, composites, cementation of bridges, root pins. Ionoseal is a light-curing glass ionomer cement. It has high tensile strength and compression resistance. Used for insulating gaskets (has good adhesion to composite materials). Vitremer is an aesthetic hybrid glass ionomer material with a triple curing mechanism (light polymerization, chemical polymerization, classical glass ionomer reaction). Used to restore the coronal part of the tooth for prosthetics, aesthetic filling and restoration.
A shining Hollywood smile from leading specialists in therapeutic dentistry. Make an appointment!
The best filling materials (as of 2022)
In the dental world, the greatest authority in the assessment of restorative materials currently belongs to the American publication The Dental Advisor. For the last 12 years (from 2010 to 2022), the highest award in the “universal composites” category has been awarded to the Japanese filling material Estelite Sigma Quick. Selection criteria: percentage of inorganic filler, amount of shrinkage, correspondence of shades to the natural color of the tooth, ease of use, radiopacity, polishability. A survey of dentists showed that they switch to Estelite more often than to other filling materials. Clinical success of Estelite is 99%.2
Among aesthetic composites in 2015, 2016, 2022, according to the same publication, the filling material from Germany, Venus Pearl, took the highest position. Its clinical effectiveness is 91%.3 In 2021, it received the title of “preferred product”. It can also be used for chewing teeth.
In 2022 and 2022, first place in the category of aesthetic composites was taken by the Harmonize material of the American manufacturer Kerr Restorative.4
Other good quality filling materials should also be mentioned. These include those who received awards in 2016-2019: Omnichroma, Brilliant EverGlow, Admira Fusion, Filtek Supreme Plus and Filtek Supreme Ultra.
Estelite – a new word in dental fillings
Quite a few patients will confirm that going to the dentist has always been a very difficult process and few could decide to do it voluntarily.
But the days when filling teeth was a painful procedure are long gone. Today, with the help of introduced innovations, new scientific approaches, high-quality materials and equipment, the prevention and treatment of the oral cavity will be painless and comfortable.
In this article we will talk about the material for filling teeth Estelite.
general information
Japanese scientists have created a special composite solution designed for affected teeth called Estelite Sigma Quick
. This is a special set of elements that have radiopaque properties and can be cured using light radiation.
In addition, this substance looks very aesthetically pleasing and does not stand out at the filling site. The polymerization process is quite fast, so the created product has become popular and used by many dental clinics around the world. Today it is included in the list of the best products among this kind of products.
Components and characteristics
The high level of quality and strength of the presented material is based on the excellent mechanical and physical properties that are inherent in the elements of the composition of this composite mass.
If we talk about filling, then Estelite largely consists of silicon-zirconium and composite filler. Its share in the total mass reaches 82%. The filling material also contains an inorganic compound, which is a spherical submicron filler. Its average particle size reaches only 0.2 microns, and the fractional composition fluctuates at the level of 0.1-0.3 microns. This element is responsible for the level of gloss and slow abrasion of the substance. The monomer base contains bis-glycedimethyl methacrylate (Bis-GMA) and triethylene glycol dimethacrylate.
The higher the degree of filling of the composite substance, the lower the level of polymerization shrinkage.
A feature of the Estelite filling material will be the use of RAP technology or a radical-enhanced polymerization activation system.
Using light radiation emitted by a photopolymerizer (peak wavelength is 470 nm), the time required for the substance to harden is reduced by almost three times compared to other materials traditionally used for filling teeth.
It is thanks to the presence of this unique system in the composition of the Estelite Sigma Quick material that the long service life of the filling is ensured. The substance is presented in syringes and in unit doses (PLT, “PreLoadedTip”).
How it works?
Dentin fragments are instantly filled with the presented preparation. This must be done so that the adhesives penetrate inside at the same level and to demineralize the composition. Only 100% occupancy allows the formation of an integral monomer structure.
In addition, the drug does not depend on whether the working area is dry or wet. The monomer adheres well and holds firmly, which is another positive quality of Estelite.
In order to calculate the material filling boundary with maximum accuracy, a special pigment is used. After the procedure, it easily disappears from the teeth and leaves no traces.
It is also worth paying attention to the fact that this drug significantly reduces the level of enamel sensitivity and promotes good strength. This is what allows it to be one level above traditional composite filling materials. Estelite is also distinguished by its versatility and ease of use.
Rating of filling materials:
| Filling material | Manufacturer | Rated by The Dental Advisor | Year of last assessment |
| Estelite Sigma Quick | Tokuyama Dental (Japan) | 99% | 2021 |
| Herculite Ultra | Kerr (USA) | 98% | 2016 |
| Tetric EvoCeram | Ivoclar Vivadent (Liechtenstein) | 97% | 2017 |
| Harmonize | Kerr (USA) | 96% | 2019 |
| Filtek Supreme Universal Restorative | 3M (USA) | 96% | 2019 |
| Filtek Supreme Plus | 3M (USA) | 96% | 2018 |
| Filtek Supreme Ultra | 3M (USA) | 96% | 2018 |
| Brilliant EverGlow | Coltene (Switzerland) | 96% | 2018 |
| Beautiful II LS | Shofu (Japan) | 96% | 2018 |
| Grandio SO | VOCO (Germany) | 96% | 2012 |
| Gradia | GC (Japan) | 96% | 2007 |
| Omnichroma | Tokuyama Dental (Japan) | 94% | 2020 |
| Beautiful II | Shofu (Japan) | 94% | 2009 |
| Admira Fusion | VOCO (Germany) | 93% | 2019 |
| Venus Pearl | Kulzer (Germany) | 91% | 2021 |
| Premise | Kerr (USA) | 91% | 2005 |