Free webinars on anti-aging medicine Learn about the features of the Anti-Age Expert International School, as well as the opportunities for improving your medical practice every day.
The webinar program also includes fascinating reviews of innovations in anti-aging medicine and analyzes of the most complex clinical cases with recommendations that really work. Find out more
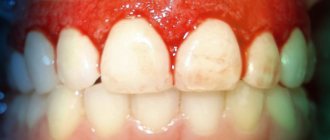
Caries
According to a study published in the medical journal The Lancet, 2.83 billion adults and children worldwide have tooth decay.
Cavities typically form when bacteria in plaque combine with sugar to form an acid that erodes enamel.
Treatment:
If you or your dentist catches tooth decay early enough, you may be able to reverse the process with fluoride medications. Otherwise, the classical treatment of caries is provided - filling.
However, if the decay gets so bad that a filling doesn't help, your dentist may crown the tooth or remove it. Again, early detection can prevent tooth decay and loss.
Gum diseases
Some form of gum disease, also called periodontal disease or periodontitis, affects nearly half of adults age 30 and older. Severity ranges from mildly swollen gums to bleeding gums and complete loss of teeth.
Most gum diseases develop in several stages:
- Plaque and tartar build up on the teeth, leading to gingivitis (inflammation of the gums).
- Gingivitis irritates the soft tissue along the gum line, which gradually leads to periodontitis.
- Periodontitis occurs when the gums separate from the teeth, forming pockets. This can lead to further gum infection, requiring treatment with antibiotics, surgery, or tooth extraction.
Treatment:
Like tooth decay, gum disease in the gingivitis stage is highly treatable if detected early. If it can't be corrected, your dentist will likely suggest scaling, a professional deep cleaning to remove all plaque from your mouth. As noted, antibiotics may also be prescribed. If periodontitis is advanced, surgery may be required.
“Periodontal disease is indeed a common problem. If such diseases are left untreated, sooner or later this will lead to the formation of deep and painful periodontal pockets, and ultimately to tooth loss. In addition, the microflora that lives in the mouth is very aggressive. Once it enters the bloodstream, it can spread and complicate the course of chronic diseases of the joints, heart, lungs, and brain, causing disruption in their functioning. The relationship between the presence of pathogenic microflora in the oral cavity and diseases such as Alzheimer's and Parkinson's disease has been proven. Getting dental plaque or the contents of periodontal pockets into the lungs when coughing can worsen the prognosis of any acute respiratory viral infection, as well as aggravate the course of COVID-19. Therefore, it is so important to monitor the condition of the periodontium, undergo preventive examinations every 6 months, and have a professional. oral hygiene, remove dental plaque, both hard and soft. When diagnosing gum disease, it is imperative to carry out timely treatment. For prevention, it is important to use simple care methods at home - brushing your teeth with high-quality toothpaste - 2 times a day, and also use dental floss, irrigators, and special rinses for additional hygiene.”
Kalashnikova Oksana Yurievna
dentist, implantologist-orthopedist, gnathologist, maxillofacial surgeon, specialist in aesthetic dentistry, candidate of medical sciences, doctor of the highest category, doctor of integrative interdisciplinary anti-aging medicine Work experience: 30 years
References
- Pomazanov, V.V. Microecological status of a person and its definition. — Prospects for the implementation of innovative technologies in medicine and pharmacy, 2022. — pp. 167-184.
- Gostry, A.V., Simonova, A.V., Mikhailova, A.N. and others. Chronic pharyngitis: etiology, pathogenesis, treatment. New approaches to assessing etiopathogenesis. - Archives of Internal Medicine, 2022. - No. 1 (45). — P.32-43.
- Man, W., Clerc, M., Pieters, W. et al. Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. – American journal of respiratory and critical care medicine, 2022. – Vol. 200(6). — P. 760-770.
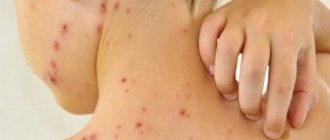
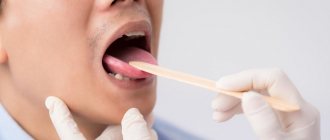
Infectious diseases
An infection of the oral cavity leads to the development of a disease in which the mucous membranes become inflamed. The pathological process can involve the teeth and gums. Symptoms of infections in the mouth are often hidden, but sooner or later they appear.
Online training in Anti-Age medicine Learn the intricacies of anti-aging medicine from anywhere in the world.
For the convenience of doctors, we have created the Anti-Age Expert online training platform: Lectures from our educational programs are consistently posted here, and are accessible 24/7. Doctors can study the materials as many times as necessary, ask questions and discuss interesting clinical cases with colleagues in special chats. Find out more
Causes of infectious diseases of the oral mucosa:
- Uncontrolled medication use.
Treatment of any disease should be under the supervision of a doctor. Improper use of antibiotics, antibacterial and other agents leads to consequences, including diseases of the oral cavity.
- HIV and AIDS.
Infectious diseases of the mucous membranes of the oral cavity often occur against their background.
- The occurrence of infections in the oral cavity
may be associated with a weakened immune system.
- Poor nutrition.
If the mucous membrane is exposed to aggressive food, it injures it. Thus, the mucous membrane becomes more vulnerable and susceptible to infection.
In addition, smoking and alcohol provoke oral diseases. Those whose bodies are dehydrated or experience hormonal imbalances also face similar problems.
- Stomatitis.
There are many bacteria living in the mouth, and due to a decrease in immunity, they are activated. Thus, an infectious disease develops. One of the most common is stomatitis. It usually develops in people who brush their teeth incorrectly or not thoroughly enough. The disease can also occur due to tonsillitis or diseases of the gastrointestinal tract. There are several types of stomatitis:
- Catarrhal.
It is manifested by swelling of the oral mucosa. As catarrhal stomatitis progresses, plaque appears on the tongue.
- Ulcerative.
In this case, the lymph nodes become enlarged. This stomatitis is manifested by dizziness and weakness. May occur in people with stomach ulcers or enteritis.
- Aphthous stomatitis.
Leads to damage to the oral mucosa, on the surface of which erosions form. Aphthous stomatitis is associated with an imbalance in the gastrointestinal tract. Signs of aphthous stomatitis: swelling of the oral mucosa, malaise, erosion in the mouth.
- Diseases caused by viruses.
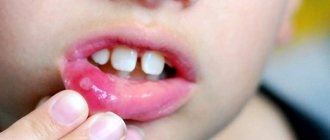
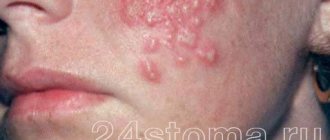
The best known oral infection is herpes, clinically known as herpes simplex virus type 1 (HSV-1). It is often found in children between 6 months and 5 years of age.
Once HSV-1 enters a child's body, he will carry the virus throughout his life. It is estimated that 50-80% of adults live with herpes simplex, either dormant or active.
The herpes virus manifests itself as rashes around the mouth. As the disease progresses, ulcers form in the mouth: they are localized on the inside of the lips and cheeks. Herpes can also infect tissues near the teeth.
After the first attack of oral herpes, the body produces antibodies to fight the virus and its effects. This way, your subsequent outbreaks of HSV-1 may not be as severe or the virus may remain dormant.
Otherwise, taking antiviral drugs may help.
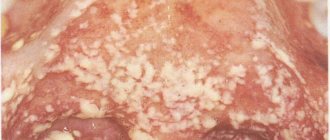
- Candidiasis.
This is a manifestation of a fungal infection. Candida fungus microorganisms live in the mouth and are activated under the influence of unfavorable factors. Candidiasis occurs in people with weakened immune systems. To avoid illness, it is necessary to strengthen the immune system and avoid hypothermia. There are several types of candidiasis:
- Pseudomembranous.
It occurs in an acute form. As the pathology progresses, the mucous membrane of the cheeks begins to dry out, and the same thing happens with the lips and tongue. A coating of curd consistency forms on the tongue. Pseudomembranous candidiasis causes discomfort when chewing and itching. The disease can occur in people with a weakened immune system, as well as against the background of blood pathologies. Other causes of pseudomembranous candidiasis are diabetes mellitus and hypovitaminosis.
- Atrophic candidiasis.
In this case, the mucous membrane of the mouth becomes red and dry. The chronic form of the disease develops in those who use dentures for a long time.
- Hyperplastic candidiasis
may be acute or chronic. In chronic cases, plaque forms on the mucous membranes, including the tongue. When you try to remove it, the mucous membrane becomes more inflamed. Brushing your teeth may cause bleeding.
- A disease that occurs due to HIV.
Secondary immunodeficiency is characterized by the active proliferation of pathogenic flora in the oral cavity. Early diagnosis will allow faster initiation of treatment and improve the prognosis of immunodeficiency.
It is important to note that diseases of the oral cavity that occur against the background of HIV often become chronic.
Oral biofilms trigger chronic inflammatory reactions that cause destructive processes. However, the characteristics of the specific reactions of the human body to biofilms are poorly understood.
Dental biofilm is a multispecies microbial community formed in fluid environments (saliva, gingival and oral fluid) with a complex structural organization, including a polymer-cellular matrix and microcolonies of microbes, regulated by numerous signaling interactions such as direct and feedback connections at the level of receptors and signaling molecules [1].
In periodontitis, during active phases of periodontal tissue destruction, gingival fluid typically contains high levels of mediators, such as proinflammatory cytokines, that promote inflammation [2].
Cytokines are short-lived, small (10-30 kDa) glycoproteins produced de novo
in response to immune stimuli. They mediate and regulate immunity, inflammation, cell growth, differentiation and hematopoiesis. They are secreted mainly by lymphocytes, monocytes and macrophages, but interact with a huge number of cells. By binding to receptors on cell membranes, they trigger second messenger complexes that carry the signal to the cell nucleus; At the same time, the expression of many genes changes. Cytokines are potent (functioning in nanomolar concentrations), redundant (many of them induce the same response), pleiotropic (individual cytokines induce multiple responses) substances that act locally between neighboring cells. They can induce a synergistic (enhancing) or antagonistic (decreasing) biological response.
According to R. Medzhitov and C. Janeway, cytokines are “molecular traffic lights” that regulate the speed and degree of immune reactions [3].
Modern research indicates that the response of gingival cells is different in the case of infection of the oral cavity (OC) with a homogeneous biofilm [4], multispecies biofilms [5] and planktonic bacteria [6].
The PR is home to residents and pathogens that maintain homeostasis with the epithelium [7]. This may be due to the fact that the innate immune system is highly active in healthy tissues. An imbalance in this system can have an impact on periodontal tissues [8].
Data from a number of foreign and domestic researchers make it possible to identify periodontopathogenic species of the 1st order (“red complex”) and 2nd order (“orange” and partially “yellow complex”), as well as a number of species that are constantly found in the PR, but the number of which increases sharply with the development of periodontitis.
First-order periodontopathogens include Aggrega-tibacter actinomycetemcomitans, Porphyromonas
gingivalis
and
Tannerella forsythia
; the possibility of their spread in the human population as an exogenous infectious agent has been proven and their tendency to intracellular parasitism in the gingival epithelium and periodontal tissues is expressed.
Second-order periodontopathogens include gram-negative anaerobic bacteria Prevotella intermedia/nigrescens, Treponema denticola, Eykenella
corrodens, Fusobacterium nucleatum, Wollinella recta
, as well as gram-positive ones -
Streptococcus intermedius, Parvimonas micra
and some representatives of the genus
Actinomyces
[9].
Biofilms and planktonic bacteria induce different signaling responses
R. Peyyala et al. [4] developed biofilm models with Streptococcus sanguinis, Streptococcus oralis, Streptococcus gordonii, Actinomyces naeslundii , F. nucleatum
and
P. gingivalis
on hard gas permeable contact lenses.
Biofilms and planktonic cultures were incubated under anaerobic conditions with the OKF4 epithelial cell line for 24 hours. P. gingivalis
significantly inhibited the production of growth regulating oncogene (Gro-1α), interleukin (IL) α, IL-6, IL-8 , transforming growth factor-α (TGF-α), fractalkine (CX3CL1), macrophage inflammatory protein α (MIP α), and interferon-α inducible protein 10 (IP-10).
In general, biofilms of all bacterial species inhibited the production of Gro-1α, TGF-α and fractalkine, while F. nucleatum
, on the contrary, stimulated a significant increase in the production of IL-1α, IL-6, IL-8 and IP-10.
A. naeslundii
biofilms also induced increased levels of IL-6, IL-8, and IP-10. Oral and biofilm planktonic streptococci weakly stimulated the release of any of these mediators by epithelial cells.
Increased amounts of acute inflammatory mediators (eg, IL-8) enhance neutrophil recruitment and potentiate the clearance of any invading microbes [10]. Biofilms of P. gingivalis
induced IL-8 gene expression, whereas IL-8 secretion did not correlate with gene expression.
This can be explained by the fact that the bacteria secrete gingipains, key virulence factors of P. gingivalis
that influence components of the human immune system [11]. Therefore, the authors believe that this model allows one to distinguish biofilms from planktonic bacteria, pathogens and commensals [4].
R. McLaughlin and A. Hoogewerf [12] also provided evidence that biofilms and planktonic bacteria respond differently to chemical mediators. Thus, biofilms of Staphylococcus aureus
, treated with 2 ng/ml IL-1β for 6 h, contained 2.5 times more cells than untreated biofilms.
No increase in bacterial growth was observed in planktonic cultures. Using flow cytometry, it was shown that
IL-1β bound to 63% of biofilm cells, but only 11% of planktonic cells.
Apparently, bacteria in the biofilm evade the human body's defense reactions, multiplying more quickly when activated protective cells release inflammatory mediators. S. Kanangat et al. [13] also showed that in vitro
S. aureus was increased in the presence of IL-1β.
Linear peptide fragments (<5 kDa) of human IL-1β also enhanced their growth. In addition, ILs modulated S. aureus
. They decreased the expression of some toxin-decoding genes and increased the expression of genes responsible for attachment to host tissues.
The results of these studies showed that biofilms and planktonic bacteria of PR induced different responses. Moreover, some types of bacteria in biofilms had high stimulating activity and, when interacting with body cell receptors, induced a response that was different from that of individual bacteria.
Single-species biofilms more effectively induced IL-8 secretion by PR epithelial cells than planktonic bacteria, with the exception of P. gingivalis
[4, 14], especially if they were part of a 3-species biofilm.
Ability of P. gingivalis
in planktonic form, inhibition of IL-8 gene expression by gingival epithelial cells was called “chemokine paralysis” [15, 16].
Mediators released in response to single-species and multi-species biofilms
A. actinomycetemcomitans is known to
is the only periodontal pathogen that is able to both recognize and bind IL-1β.
In clinical strains of this species, a physiological response to cytokines was observed, expressed in a decrease in metabolism and an increase in biofilm mass [17]. A. actinomycetemcomitans
biofilms cultured in an organotypic gingival mucosa model bound IL-1β, but the use of antibiotics during their coculture inhibited its binding.
Moreover A. actinomycetemcomitans
appears to uptake IL-1β, as this cytokine has been detected in the extracellular space and cytoplasm of these bacteria [18].
Intracellular proteins (the three-dimensional form of the adenosine triphosphate β-synthase subunit and the bacterial histone-like protein HU) can interact with human IL-1β [17, 18]. Interaction of internalized IL-1β with a key protein in cellular energy production and the genomic DNA condensing protein HU may explain the physiological responses of A. actinomycetemcomitans
.
These results suggest that viable A. actinomycetemcomitans
have a specific mechanism for uptake of IL-1β.
The genome of A. actinomycetemcomitans
encodes an outer membrane lipoprotein specific to
Pasteurellaceae
and responsible for interaction with IL-1β [18].
When the viability of cells in biofilms decreases, their ability to bind IL-1β is blocked, which leads to the leakage of IL-1β into the culture medium. In some A. actinomycetemcomitans
, IL-1β is localized to the outer edge of the nucleoid.
Close contact with living biofilms of A. actinomycetemcomitans
reduced proliferation and apoptosis of human gingival keratinocytes.
The authors suggest that A. actinomycetemcomitans
may disrupt the crucial first step of local inflammation in periodontitis by binding and internalizing IL-1β [17].
Multispecies biofilms have unique characteristics, and depending on the species composition, they can be associated with healthy periodontium, gingivitis or periodontitis, which was confirmed using scanning electron microscopy in combination with polymerase chain reaction [19].
P. gingivalis, T.
forsythia
and
T. denticola
are periodontal pathogens of the so-called “red complex”.
They are associated with subgingival biofilm at sites of damage during periodontitis. G. Belibasakis et al. [10] showed that when cultivating a multilayer organotypic culture of human epithelial cells with a 10-species biofilm - Campylobacter rectus (OMZ 697), F. nucleatum (OMZ 596), P. gingivalis ATCC 33277T (OMZ 925), P. intermedia ATCC 25611T (OMZ 278), T. forsythia OMZ1047, T. denticola ATCC 35405T (OMZ 661), Veillonella dispar ATCC 17748T (OMZ 493), Actinomyces oris (OMZ 745), Streptococcus anginosus (OMZ 871) and S. oralis SK 248 (OMZ 607)) or 7-species, in which there were no periodontopathogens of the “red complex,” after 3 hours the expression of IL-8 genes increased equally regardless of the species composition of the biofilm. When adding periodontal pathogens of the “red complex”, the expression of IL-8 genes increased 3 times. After 24 h, IL-8 secretion was reduced to 50% of the level in control cells cultured on saliva-treated hydroxyapatite. But this did not occur in the absence of periodontal pathogens of the “red complex”. The authors concluded that periodontal pathogens of the “red complex” in the biofilm regulate the chemotactic response differently. Other authors have also shown that IL-8 expression is induced by T. denticola
,
P. gingivalis
,
T. forsythia
, and their associations [20].
In a 9-species system without T. denticola
There was an increase in the secretion of IL-1, IL-6 and IL-8 after 4 hours and a decrease after 24 hours [5].
It can be assumed that both polymicrobial associations and P. gingivalis
,
T. denticola
and
T. forsythia
may regulate the IL-8 chemoattractant response of epithelial cells, having the potential to disrupt host-microbe homeostasis, possibly as a strategy to manipulate the local innate immune system response and increase their chance of survival in the subgingival niche.
These results are consistent with the data that biofilms of one species, F. nucleatum
,
A. naeslundii
,
S. gordonii
and
S. oralis
also increased the expression of IL-8 genes in epithelial cells of the PR after 6 hours of
in vitro
, while
S. sanguinis
had no effect, and
P. gingivalis
even decreased their expression [21].
A biofilm consisting of S.
gordonii
,
A. naeslundii
and
F. nucleatum
induced an increase, and a biofilm including
S. gordonii, F. nucleatum
and
P. gingivalis
decreased the secretion of IL-8 [22].
This may indicate that P. gingivalis
is able to reduce the chemoattractant response of epithelial cells [10].
R. Peyyala et al. [22] identified specific mediators whose levels were significantly higher when stimulated by multispecies biofilms compared to what would be expected from a simple additive effect of monobiofilms. Finally, many biofilms exhibit inhibitory effects or do not alter normal background levels of mediators, translation and secretion, and/or degrade various mediators in biofilm model systems. This often contrasts with the levels of mediators expected from stimulation with monobiofilms or infection with planktonic bacteria [4].
In a multispecies biofilm system on hydroxyapatite disks cultured with epithelial, periodontal ligament, or dental pulp cells, significant increases in apoptosis and degradation of IL-1β, IL-6, and IL-8 were observed. Biofilms represented by some species significantly increased the content of a number of mediators, while biofilms of P. gingivalis
had the opposite effect even on the basal production of cytokines/chemokines [5].
In addition, P. gingivalis
to inactivate tumor necrosis factor (TNF-α) [23].
Biofilms consisting of S.
gordonii, S. oralis, S. sanguinis, F. nucleatum
and
P. gingivalis
to induce higher levels of IL-1α and exhibit synergistic stimulatory activity compared with the additive response of 3 individual bacterial species.
Only biofilms of S. gordoni/A.
naeslundii and A. naeslundii/F. nucleatum induced higher levels of IL-6 secretion than the control.
When cultivated with S. gordoni/A.
naeslundii/F. nucleatum was the first to secrete IL-8, although its level was not higher than the putative compositional level induced by monobiofilms [24].
Epithelial cells recognize resident and pathogenic microbes
Recognition of both residents and pathogens can initiate an innate immune response through toll-like receptors (TLRs) [25]. E. Millhouse et al. [26] revealed clear differences in the response of epithelial cells after infection by commensals and pathogens. These data are consistent with those of B. Dickinson et al. (2011) and Y. Hasegawa et al. (2007), who studied in vitro
reactions of the gingival epithelium to pathogens and residents [27, 28].
Residents of P.R. S. gordonii
, as well as
F. nucleatum,
induced the transcriptome of epithelial cells less significantly compared to the periodontopathogens
P. gingivalis
and
A. actinomycetemcomitans
. A limitation of this work was that a suspension of bacteria was cultured with epithelial cells.
Other researchers have also identified different responses of epithelial cells (secretion of cytokines and chemokines) to pathogen and resident biofilms [4]. They also determined lower levels of IL-8 and IL-1α production on P. gingivalis
and
Streptococcus
spp
.
R. Peyyala and J. Ebersole believe that the ability of different bacterial species in the dental biofilm to induce different cytokine profiles in gingival epithelial cells may reflect their individual virulence or resident status [6]. So, P. gingivalis
induced high levels of IL-1β,
A. actinomycetemcomitans
- IL-8,
F. nucleatum
- IL-1β and IL-6,
S. gordonii
caused a minimal chemokine response.
All periodontopathogens of the “red complex” are capable of synergistically colonizing gingival epithelial cells. There was a tendency for co-localization of P. gingivalis
and
T. denticola.
In the absence of periodontopathogens of the “red complex”, the gingival epithelium is colonized by streptococci, mainly
S. oralis
. Based on this antagonism, the authors believe that the “red complex” bacteria can regulate the virulence of the biofilm, which plays a role in the pathogenesis of periodontitis [29].
Mixed pathogen biofilms and P. gingivalis
possess a huge number of virulence factors that can induce an innate immune response.
P. gingivalis
contacts epithelial cells using fimbriae and gingipains and penetrates the cells [30].
Lipopolysaccharide and its lipid component, P. gingivalis
, induce a strong response from the human immune system because it binds to the TLR complex, promoting the secretion of proinflammatory cytokines by epithelial cells and other cell types [31].
P.
gingivalis gingipains destroy cytokines and the host cytokine receptor network, including IL-1β, interferon-γ (IFN-γ), and TNF-α [32].
This may explain why the secretion of these cytokines and chemokines does not correlate with gene expression. All these factors determine the pathogenic potential of P. gingivalis
. Epithelial cells do not completely protect the host from the action of a biofilm containing periodontopathogenic bacterial species.
In a series of elegant experiments, T. Maekawa et al. [33] showed that P. gingivalis
inhibits bacterial killing by neutrophils and at the same time maintains a strong cytokine pro-inflammatory response.
These processes are accompanied by coactivation of TLR-2 and C5a receptors of neutrophils. It is important to note that this work provides evidence, at least in a mouse model, that activation of phosphatidylinositol 3-kinase by P. gingivalis
not only enhances its own resistance to phagocytosis, but also that of another dental biofilm associate,
F. nucleatum.
According to S.S. Afanasyeva et al. [34], ILs and IFNs are able to react directly with microbes, changing their growth rate and biological properties, including sensitivity to antibacterial drugs. Thus, it was found that TNF-α and IFN-γ increased the sensitivity of S. aureus and Enterobacter cloacae
and
Escherichia coli
to benzylpenicillin and tetracycline, and IFN-α2, on the contrary, reduced the sensitivity of these bacteria to antibiotics, i.e., provoked resistance, even blocking the combined action of TNF-α and IFN-γ.
It is currently not known exactly how Streptococcus mitis
interact with the innate immune system.
S. mitis
has previously been shown to be significantly tolerant of human β-defensins-2 and other antimicrobial peptides (AMPs) [35, 36].
S. mitis
can also modulate the expression of the proinflammatory chemokine IL-8 [37].
Based on this, we can assume that S. mitis,
as a beneficial commensal, is capable of complementing the host immunity by maintaining tissue homeostasis.
Virulence of Candida albicans
higher in mature mixed multispecies biofilms cultured on reconstituted human epithelial cells (RHOE) than in single-species biofilms.
In this case, an increase in the secretion of IL-18, lactate dehydrogenase activity and invasiveness of C. albicans is observed.
In response to
C. albicans
, PR epithelial cells produce large amounts of IL-6, IL-8, and TNF-α.
This indicates that cytokines play a significant role in the control of PR infections [38, 39]. After exposure to Candida parapsilosis
, human gingival epithelial cells express high levels of TLR-2, TLR-4, but not TLR-9 mRNA.
Increased levels of cytokines and AMPs may explain the inhibition of the growth of C. parapsilosis
on gingival epithelial cells [40, 41].
In summary, all of these studies showed that epithelial cells respond differently to pathogens and commensals. The exact mechanism of these processes is not fully understood.
It is known that the formation of biofilms makes a significant contribution to the formation of resistance in bacteria to antibiotics and factors of innate immunity of the host organism [42, 43]. Mechanisms of biofilm resistance to host defenses may include changes in gene expression that mediate reactivity to chemical mediators. Since biofilms are more similar to bacterial growth in vivo
and participate in the formation of infections, it can be assumed that they, but not planktonic cells, should have responded to cytokines.
In addition to various types of bacteria, herpes viruses and yeasts found in subgingival biofilms can recognize and bind cytokines produced by the host [44].
Even though important scientific results have been obtained in the field of biofilm research, biofilm control remains an unresolved problem and is a central focus of modern research.
Thus, the innate immune system is characterized by a differentiated cytokine response to PR bacteria.
The significance of these mechanisms for wide clinical practice determines the need to introduce adequate and reliable methods for assessing the components of IL systems, defensins and their receptors, which can be reproduced in the clinical laboratory of medical institutions.
The authors declare no conflict of interest.
Oral cancer
Oral cancer most often affects the tongue, tonsils, gums and oropharynx. Since its various types often do not cause obvious signs and symptoms in the early stages, regular dental examinations are the most important method of detecting them.
Your dentist may also test you for oral cancer during your exam, especially if you have any of these symptoms:
- Persistent pain in the mouth or lip;
- Red or white spot in the mouth;
- Loose teeth;
- Painful swallowing, constant pain in the mouth or ears.
Treatment:
Depending on the type and stage of oral cancer, treatment may include a combination of surgery, radiation therapy and chemotherapy.
Stomatitis
This is a group of diseases characterized by inflammation of the oral mucosa with hyperemia, swelling, and an increase in the amount of mucus in the oral cavity.
Depending on the severity and depth of the lesion, even ulcers or foci of necrosis may form in the oral cavity, sharply disrupting the general condition - fever, weakness, anxiety, refusal to eat. There are many causes of the disease: mechanical, chemical, thermal, bacterial factors. Often the cause of the disease in infancy is contaminated nipples, toys and other objects that fall into the child’s mouth. Stomatitis often develops as a result of infectious diseases (measles, scarlet fever, influenza, whooping cough, etc.). The mucous membrane of the oral cavity acquires a bright red color, becomes swollen, and tooth marks are visible on the mucous membrane of the cheeks and tongue. Saliva becomes viscous and viscous. The mucous membrane is covered with a whitish coating. The tongue is dry, swollen, often with a brown tint, chewing is painful. The duration of the disease is from 1 to 3 weeks, the prognosis is favorable.
A general preventive rule for children and adults is to maintain good oral hygiene.
Prevention
Maintaining oral health is not difficult if you follow simple recommendations:
- Eat a balanced diet.
The diet should contain a sufficient amount of protein foods, as well as foods containing amino acids, trace elements and vitamins. Carbohydrate foods, on the contrary, are best consumed in small quantities.
- Careful oral hygiene.
It is important, for example, to use dental floss and brush your teeth with toothpaste or gel twice a day.
- Use of vitamins and nutritional supplements (as prescribed by a doctor).
This will make up for deficiencies in the body that affect oral health.
- Regular visits to the dentist.
You should visit your dentist for a checkup at least once a year. Even if there are no complaints.
Brief conclusions
- The most common oral diseases are tooth decay, gum disease, infectious diseases and oral cancer.
- Caries and gum disease are highly treatable in the early stages.
- The causes of infectious diseases of the oral cavity vary - from viruses to poor diet and uncontrolled use of medications.
- For prevention, it is important to review “life hygiene” and regularly visit the dentist.
Anti-Aging Medicine Seminars Gain knowledge based on evidence-based medicine first-hand from the world's leading experts. As part of the Anti-Age Expert Modular School, in-person two-day seminars are held every month, where the intricacies of anti-age medicine are revealed to doctors of more than 25 specialties
Find out more